Abstract
The aim of this study was to compare the time-to progression and overall survival (OS) in patients with metastatic breast cancer (MBC) with and without deleterious BRCA1/2 mutations. 195 women with MBC who were referred for BRCA genetic testing between 1997 and 2011 were included in the study. Logistic regression models and Cox proportional hazards models were fit to determine the associations between clinical variables and outcomes. Of 195 women with MBC, 21 % (n = 41) were positive for BRCA1/2 mutations. The number of metastatic sites at the time of metastatic disease was not different between BRCA1 versus BRCA2 carriers versus non-carriers (P = 0.77). The site of first metastasis was visceral-only in 70 % of BRCA1 carriers compared to 9 % in BRCA2 carriers and 37 % in non-carriers (P = 0.001). Median follow-up was 2.8 years. BRCA non-carriers and BRCA2 carriers had a longer time-to progression and OS compared to BRCA1 carriers (median time-to progression = 1.3 vs. 0.9 vs. 0.7 years; P = 0.31, and median OS = 4.88 vs. 4.94 vs. 1.34 years; P = 0.0065). In a multivariate model, no association was identified between BRCA positivity and time-to-event outcomes (P > 0.28). In addition, patients with triple-negative MBC carried a poorer prognosis irrespective of their BRCA status (P = 0.058 and P = 0.15 for the interaction term of BRCA status and triple-negative for time-to progression and OS, respectively). Our data indicate that BRCA1 carriers diagnosed with MBC have worse outcomes compared to BRCA2 carriers and non-carriers. However, the differences in outcome did not reach statistical significance likely due to small sample sizes.
Keywords: BRCA mutation, Chemotherapy, Metastatic breast cancer, Recurrence, Survival
Introduction
Metastatic breast cancer (MBC) comprise only 6 % of all newly diagnosed breast cancer cases [1]; however nearly 30 % of women initially diagnosed with early stage breast cancer eventually develop metastatic disease [2]. Despite advances in the treatment of MBC, estimated 5-year overall survival (OS) rates ranges from 21 to 40 % [3, 4].
Breast cancer is no longer viewed as a homogenous disease process, but rather as a compilation of reproducibly identified intrinsic subtypes as defined by microarray gene expression (luminal A, luminal B, basal-like, and human epidermal growth factor receptor 2 [HER2]-enriched sub-types, normal like), each characterized by unique clinico-pathologic features and a distinct prognosis [5–7]. Moreover, gene expression studies have identified subtype-specific predilection to distant metastatic sites [8]. The bone is a common site of first metastases in women with estrogen receptor (ER)-positive and HER2-normal breast cancer, whereas basal-like and HER2-positive breast cancer are more likely to recur in visceral sites, including the central nervous system [9]. It was shown that a high risk of developing visceral metastases shortly after diagnosis was responsible in large part for the adverse prognosis associated with the triple-negative (TN) or basal-like phenotype [10].
It has been shown that more than 75 % of breast cancer in BRCA1 carriers have a TN phenotype, a basal-like phenotype, or both [11]; despite this, most studies showed a similar outcome for BRCA mutation carriers compared to age-matched women with sporadic breast cancers [12–18] in neoadjuvant and adjuvant settings. Up to date, there are limited data on the association of BRCA-associated breast cancer with specific clinical behaviors in the MBC setting. Tan et al. evaluated the clinical impact of germ-line BRCA1/2 mutations in patients with epithelial ovarian cancer (EOC) on responses to first and subsequent lines of chemotherapy, treatment-free interval between each line of therapy, and OS, and observed that BRCA-positive EOC patients had better outcomes than non-hereditary EOC patients [19]. In another study conducted among women with high-grade serous ovarian cancer, BRCA2 mutation, but not BRCA1 deficiency, was found to be associated with improved survival, improved chemotherapy response, and genome instability compared with BRCA wild-type [20]. Likewise Alsop et al. [21] reported improved survival outcomes in patients with ovarian cancer carrying germ-line mutations. In that study, patients carrying mutations more frequently responded to both platinum and non-platinum-based regimens than mutation-negative patients.
We conducted this study to analyze the differences in the spectrum of metastasis observed during the course of the metastatic disease, and in time-to progression (TTP), or OS between BRCA mutation-associated MBC and non-BRCA mutation-associated MBC. Our secondary objective was to identify potential prognostic variables occurring in patients with MBC.
Materials and methods
Patient population and data collection
The Breast Cancer Management System database of the University of Texas MD Anderson Cancer Center (MDACC) identified 195 women who were diagnosed with MBC, and were referred for genetic counseling for mutations in the BRCA1 and BRCA2 genes, between 1997 and 2011. Patients with BRCA mutation with uncertain significance were excluded from the analysis.
A distant metastases was classified as a “bone-only” if there was evidence of metastases to bone-only; “visceral” if there was radiological evidence of metastases to viscera (including lung, liver and/or brain), or involvement of soft tissues including lymph nodes and skin; and “mixed” if there was evidence of both bone and visceral metastases at the same time.
This study was approved by the MDACC Institutional Review Board. The prospectively collected data included patient and tumor characteristics at the time of original breast cancer diagnosis, type of chemotherapy or endocrine therapy received, recurrence and survival information, family history of breast cancer or ovarian cancer, and the BRCA gene status. After referral and genetic testing for a BRCA mutation, patients have continued their follow-up and treatment at MDACC. For patients who were treated primarily at another hospital where the primary diagnosis was performed, the data on treatment have been acquired through outside records.
During the study period, patients with ER-negative metastatic disease or patients with extensive liver or lung metastases from ER-positive tumors were treated with chemotherapy. The chemotherapy regimens given were at the discretion of the patient and the oncologist. Patients with bone metastases or low volume liver and lung metastases from ER-positive tumors received a trial of endocrine therapy.
Pathologic assessment and mutation analysis
All pathologic specimens were reviewed by breast pathologists at MDACC. Initial clinical and pathologic stage of all patients were based on the sixth edition of the American Joint Committee on Cancer (AJCC) staging criteria [22]. Tumor grade was defined according to the modified Black’s nuclear grading system [23]. Nuclear staining ≥10 % of ER or progesterone receptor (PR) was considered strongly positive on immunohistochemistry (IHC). We need to mention that in our institution, a 10 % threshold was used to report a positive result of ER until 2010; however since then we report a tumor positive if only 1 % of the cells stain for ER/PR antibodies. Since the patients included in the analysis dates back to 1997, we selected a uniform threshold for all study patients as the treatments they had received in 1990s were based on 10 % threshold.
Human epidermal growth factor receptor 2 positivity was defined as 3+ receptor overexpression on IHC staining and/or gene amplification by fluorescence in situ hybridization (HER-2/CEP 17 ≥ 2 were considered as amplified).
BRCA genetic testing was performed using germline DNA by Myriad Genetics Laboratories, Inc (Salt Lake City, UT) and the test results were categorized as either positive or negative for a BRCA deleterious mutation.
Statistical analysis and outcome measures
Patient demographics, clinical characteristics were compared between the three groups by BRCA status (non-carrier vs. BRCA1 carrier vs. BRCA2 carrier), using χ2 tests for categorical variables and t-tests/ANOVA or the counterparts of nonparametric approaches for continuous variables. For patients who presented with stage 1–3 breast cancer initially, we also calculated time-to-recurrence (TTR) which was defined as time from the definitive surgery until the first date of documented locoregional and/or distant metastatic disease. For the whole study population, time to progression (TTP) was calculated from the time of first documented distant metastatic disease to disease progression (encompasses one type/line of therapy for meta-static disease) or to the time of last follow-up. OS was calculated from the time of first documented distant metastatic disease to the time of death from any cause or last follow-up. Patients not experiencing the relevant end points were censored at last follow-up.
Survival outcomes were estimated with the Kaplan– Meier product-limit method. Cox proportional hazards models were fitted to determine the association of BRCA mutation status with time to event outcomes after adjustment for significant patient and clinical characteristics identified in univariate analyses. P values of ≤ 0.05 were considered statistically significant; all tests were two-sided. Statistical analysis was carried out using SAS 9.1.3 (SAS Institute Inc., Cary, NC) and S-Plus 8.0 (Insightful Corporation, Seattle, WA).
Results
Patient demographics and clinical characteristics
One hundred and ninety-five patients were identified for this analysis, of whom 21 % (n = 41) were BRCA mutation carriers; and 15 % of those (n = 30) were BRCA1 mutation carrier, and 6 % (n = 11) were BRCA2 mutation carrier. The prevalence of BRCA mutations with regard to patient demographics and clinical characteristics is summarized in Table 1. The median ages at diagnosis of primary breast cancer and of distant metastatic disease were not different between the BRCA carriers and non-carriers (P ≥ 0.16). Compared to non-carriers and BRCA2 carriers, BRCA1 carriers were more likely to have higher nuclear grade 3 tumors (87 vs. 65 % vs. 78 %; P = 0.03), ER-negative (63 vs. 37 % vs. 30 %; P = 0.03), PR-negative (87 vs. 51 % vs. 44 %; P = 0.0005), and TN tumors (58 vs. 23 % vs. 17 %; P = 0.002) compared to BRCA2 carriers and non-carriers. Other disease characteristics were not different between the three groups (P ≥ 0.11).
Table 1.
Patient demographics and baseline clinical characteristics by BRCA groups
| BRCA non-carrier N = 154 (%) |
BRCA1 carrier N = 30 (%) |
BRCA2 carrier N = 11 (%) |
P | |
|---|---|---|---|---|
| Median age at diagnosis of primary | ||||
| ≤38.6 | 74 (48) | 18 (60) | 5 (45.5) | 0.48 |
| >38.6 | 80 (52) | 12 (40) | 6 (54.5) | |
| Median age at diagnosis of metastases | ||||
| ≤41.5 | 74 (48) | 19 (63) | 5 (45.5) | 0.30 |
| >41.5 | 80 (52) | 11 (37) | 6 (54.5) | |
| Race | ||||
| Black | 15 (9.9 %) | 4 (13.3 %) | 2 (18.2 %) | 0.56 |
| White | 23 (15.2 %) | 23 (76.7 %) | 9 (81.8 %) | |
| Others | 113 (74.8 %) | 3 (10 %) | – | |
| Tumor size of primary tumor | ||||
| T1 | 42 (29.4 %) | 2 (7.1 %) | 4 (36.4 %) | 0.15 |
| T2 | 64 (44.8 %) | 17 (60.8 %) | 4 (36.4 %) | |
| T3 | 18 (12.6 %) | 3 (10.7 %) | 1 (9.1 %) | |
| T4 | 19 (13.3 %) | 6 (21.4 %) | 2 (18.2 %) | |
| Nodal status | ||||
| N0 | 48 (35.3 %) | 7 (27 %) | 4 (44.4 %) | 0.60 |
| N1 | 48 (35.3 %) | 9 (34.6 %) | 4 (44.4 %) | |
| N2 | 21 (15.4 %) | 3 (11.5 %) | 1 (11.1 %) | |
| N3 | 19 (14 %) | 7 (27 %) | – | |
| Clinical stagea | ||||
| M0 | 113 (78.5 %) | 25 (86.2 %) | 7 (70 %) | 0.46 |
| M1 | 31 (21.5 %) | 4 (13.8 %) | 3 (30 %) | |
| Nuclear grade | ||||
| 1 | 1 (0.7 %) | 1 (3.3 %) | – | 0.03 |
| 2 | 50 (34.7 %) | 3 (10 %) | 2 (22.2 %) | |
| 3 | 93 (64.6 %) | 26 (86.7 %) | 7 (77.8 %) | |
| ER- status | ||||
| Negative | 53 (37.3 %) | 19 (63.3 %) | 3 (30 %) | 0.03 |
| Positive | 89 (62.7 %) | 11 (36.7 %) | 7 (70 %) | |
| PR-status | ||||
| Negative | 72 (51.4 %) | 26 (86.7 %) | 4 (44.4 %) | 0.0005 |
| Positive | 68 (48.6 %) | 4 (13.3 %) | 5 (55.6 %) | |
| HER2-status | ||||
| Negative | 90 (75 %) | 24 (92.3 %) | 5 (71.4 %) | 0.11 |
| Positive | 30 (25 %) | 2 (7.7 %) | 2 (28.6 %) | |
| Triple-negative | ||||
| No | 92 (76.7 %) | 11 (42.3 %) | 5 (83.3 %) | 0.002 |
| Yes | 28 (23.3 %) | 15 (57.7 %) | 1 (16.7 %) | |
| Site of first metastases | ||||
| Bone-only | 46 (29.9 %) | 3 (10 %) | 6 (54.6 %) | 0.001 |
| Visceral-only | 51 (33.1 %) | 21 (70 %) | 1 (9.1 %) | |
| Mixed | 57 (37 %) | 6 (20 %) | 4 (36.4 %) | |
| Number of metastatic sites | ||||
| 1–2 | 24 (80.0 %) | 9 (81.8 %) | 115 (74.7 %) | 0.77 |
| ≥3 | 6 (20.0 %) | 2 (18.2 %) | 39 (25.3 %) |
ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor-2
Clinical stage at initial diagnosis
The median time between breast cancer diagnosis and BRCA genetic testing was 25.5 months. To avoid potential ascertainment (Neyman) bias due to time interval between cancer diagnosis and genetic testing, we did an additional survival on a subset of 177 patients who had their genetic counseling and testing within 12 months of their diagnosis. The distributions of clinical and pathologic characteristics were similar in this subgroup as compared with the entire series (data not shown).
Of 195 women analyzed in this study, 19 % (n = 38) had distant metastatic disease at the time of initial breast cancer diagnosis. Forty-seven (24 %) patients presented with ≥3 different sites of metastasis. Number of metastatic sites at the time of diagnosis was not different between BRCA1 versus BRCA2 carriers versus non-carriers (P = 0.77). The site of first metastasis was visceral-only in 70 % of BRCA1 carriers versus 33 % of non-carriers versus 9 % of BRCA2 carriers (P = 0.001). Visceral sites of involvement were not different between the three groups, including liver (17 vs. 36 % vs. 29 %; P = 0.28), and brain (10 vs. 0 % vs. 10 %; P = 0.8) in BRCA1 versus BRCA2 carriers versus non-carriers.
Treatment modalities for MBC
For four women, endocrine therapy could not be analyzed. Patients with ER-positive tumors received endocrine therapy regimens as follows: among BRCA carriers (n = 10), 80 % (n = 8) received up to two regimens, 20 % (n = 2) received up to three to four regimens. Among non-carriers (n = 69), 68.1 % (n = 47) received up to two regimens, and 29 % (n = 20) received up to three to four regimens. The median (range) number of lines of endocrine therapy used between non-carriers versus BRCA1 versus BRCA2 carriers were statistically different [1 (0–7), 0 (0–3), and 1 (0–4), respectively; P = 0.002; Table 2].
Table 2.
Treatment characteristics of metastatic breast cancer by BRCA groups
| BRCA non-carrier | BRCA1 carrier | BRCA2 carrier | P | |
|---|---|---|---|---|
| Metastatic ET | ||||
| Median Duration (range; months) of First-line ET (N) | 10.35 (0.69–91.27) | 7.66 (3.02–60.02) | 4.27 (1.84–58.45) | 0.68 |
| (35) | (5) | (7) | ||
| Median Duration (range; months) of Second-line ET (N) | 10.79 (1.84–91.27) | 7.39 (7.13–7.66) | 30.14 (1.84–58.45) | 0.84 |
| (18) | (2) | (2) | ||
| Median Duration (range; months) of Third-line ET (N) | 10.79 (1.84–57.99) | 7.39 (7.13–7.66) | 1.84 (N/A) | 0.23 |
| (12) | (2) | (1) | ||
| Median (range) Number of Lines of ET (N) | 1 (0–7) | 0 (0–3) | 1 (0–4) | 0.002 |
| (152) | (28) | (11) | ||
| Metastatic CT | ||||
| Median Duration (range; months) of First-line CT (N) | 4.91 (1.54–62.03) | 4.14 (1.38–9.33) | 4.80 (4.04–24.94) | 0.43 |
| (56) | (19) | (8) | ||
| Median Duration (range; months) of Second-line CT (N) | 6.14 (1.61–33.68) | 6.64 (2.1–7.23) | 4.99 (4.04–24.94) | 0.99 |
| (19) | (3) | (3) | ||
| Median Duration (range; months) of Third-line CT (N) | 6.44 (2.07–33.68) | 4.67 (2.1–7.23) | 4.52 (4.04–4.99) | 0.49 |
| (13) | (2) | (2) | ||
| Median (range) Number of Lines of CT (N) | 2 (0–10) | 2 (1–5) | 2 (0–7) | 0.47 |
| (152) | (28) | (11) |
ET endocrine therapy, CT chemotherapy
During the course of their metastatic disease, the majority of patients (86.9 %; n = 166/191) received either platinum- or taxane-based chemotherapy regimens, and no significant differences in the use of chemotherapy regimens were found between BRCA carriers and non-carriers (P = 0.33). The first-line chemotherapy regimens for metastatic disease included capecitabine, CMF, taxol ± bevacizumab, abraxane ± bevacizumab, FAC/FEC, AC, TC, TAC, TCH, taxol or taxotere ± bavacizumab, ixabepilone ± capecitabine, gemcitabine ± carboplatin, vinorelbine ± becavizumab. Of 38 BRCA carriers, 84.2 % (n = 32) received up to three chemotherapy regimens, and 13.2 % (n = 5) received four to six regimens. Of 127 non-carriers, 71.7 %(n = 91) received up to three chemotherapy regimens, 21.3 % (n = 27) received four to six regimens, and 7.1 % (n = 9) received more than six subsequent regimens. Table 2 summarizes the median duration of first-, second-, and third-line endocrine therapy and chemotherapy in BRCA carriers and non-carriers.
Among women whose tumors overexpressed HER2, the use of trastuzumab (P = 1.0) or lapatinib (P = 0.11) between BRCA carriers and non-carriers were not statistically different.
Probability of survival
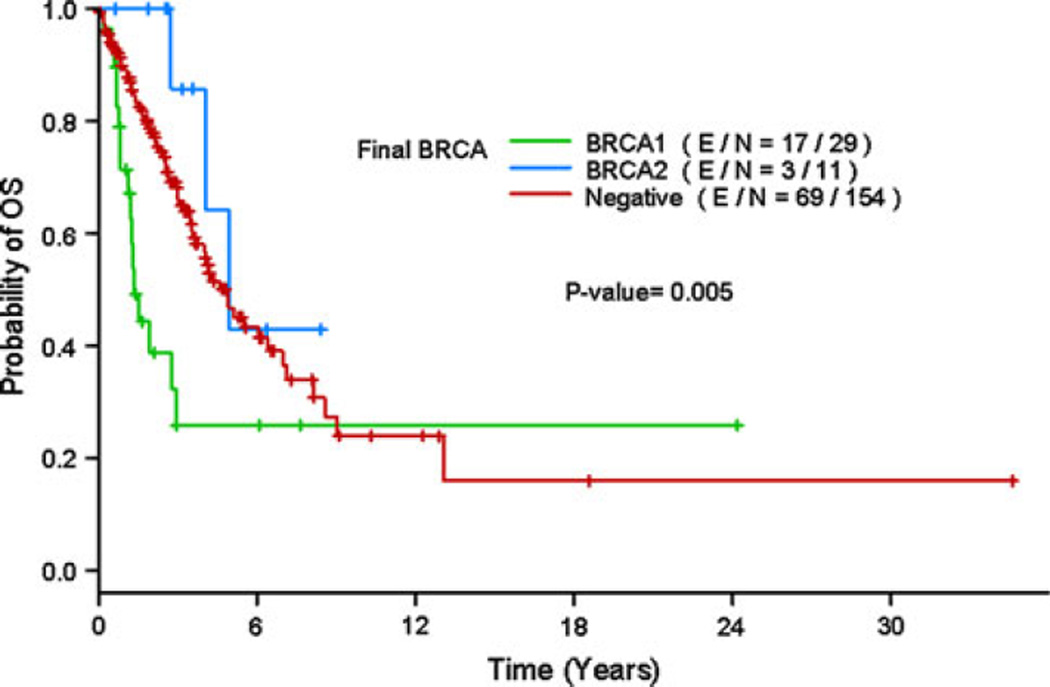
The median follow-up times since the start of diagnosis of the metastatic disease for survivors were 2.8 years (range 0.05–34.6) for all patients, 1.5 years (range 0.7–24.2) for BRCA1 mutation carriers, 2.9 years (range 0.6–8.4) for BRCA2 mutation carriers and 2.9 years (range 0.05–34.6) for non-carriers. For the whole study population, the median TTP was 1.1 years (95 % CI: 0.9–1.4 years), and the median OS was 4.2 years (95 % CI: 3.5–6.1 years). BRCA non-carriers and BRCA2 carriers had a longer TTP and OS compared to BRCA1 carriers (median TTP = 1.3 vs. 0.9 vs. 0.7 years; P = 0.31, and median OS = 4.9 vs. 4.9 vs. 1.3 years; P = 0.0065). During follow-up, 17 of 30 BRCA1 carriers (56.7 %), 3 of 11 BRCA2 carriers (27.3 %) and 69 of 154 non-carriers (44.8 %) had died. The estimated 5-year OS rates as of diagnosis of metastatic disease were 46.8 % in the BRCA non-carrier group versus 25.9 % in the BRCA1 carrier versus 42.9 % in the BRCA2 carrier group (Fig. 1).
Fig. 1.
Kaplan–Meier estimate of overall survival by BRCA status
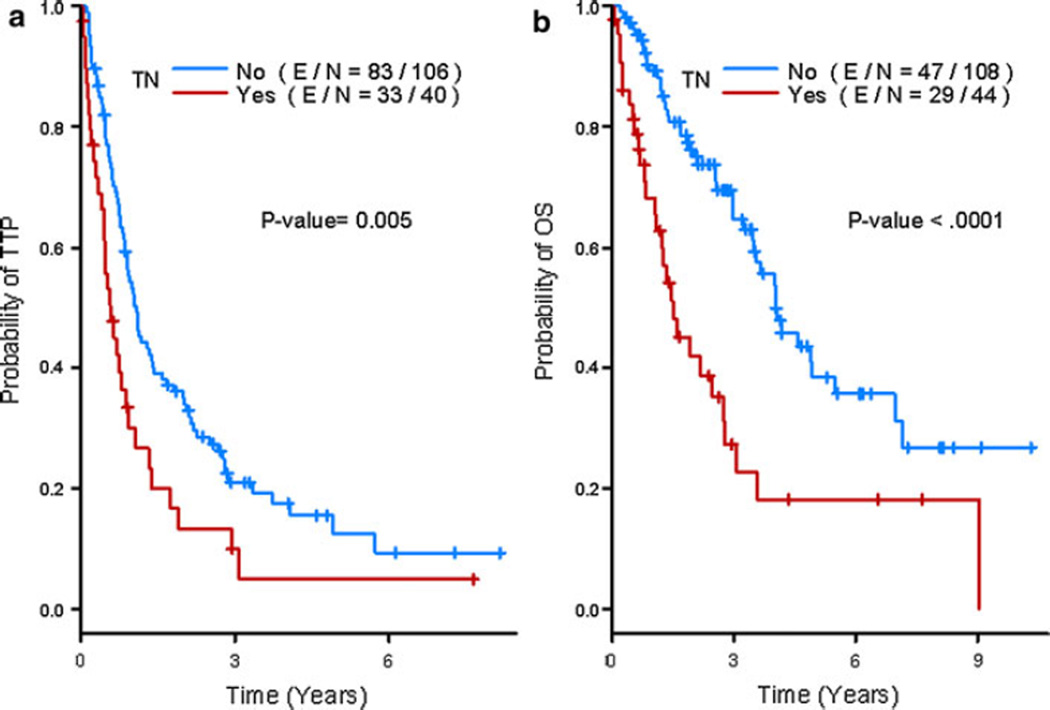
Patients with TN breast cancer had significantly shorter TTP and OS than those with other subtypes of breast cancer (median TTP = 0.6 vs. 1.1 years; P = 0.0052, and median OS = 1.5 vs. 4.0 years; P < 0.0001). The 5-year TTP estimates as of diagnosis of metastatic disease were 5 and 12.5 %; and 5-year OS estimates were 18.2 and 38.5 % (Fig. 2b) for patients with TN breast cancer and those with other forms of breast cancer, respectively. In addition, patients with TN MBC carried a poorer prognosis irrespective of their BRCA status (P = 0.058 and P = 0.15 for the interaction term of BRCA status [positive vs. negative] and TN for TTP and OS, respectively).
Fig. 2.
Kaplan–Meier estimates of time-to-progression (a) and overall survival (b) by Triple-negative status
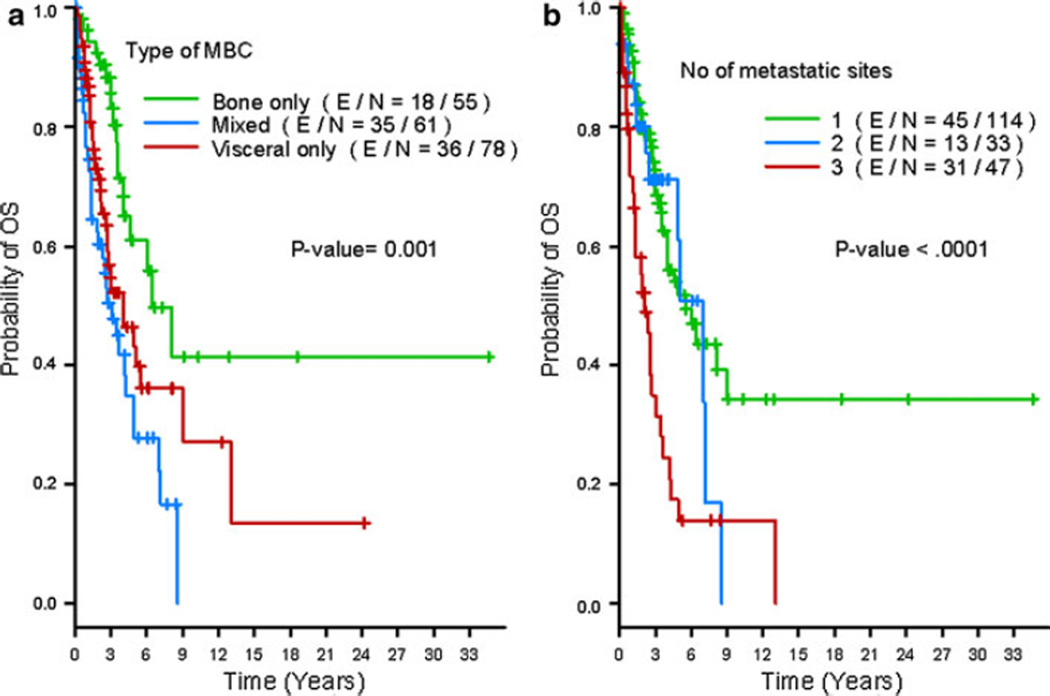
Visceral and mixed involvement at initial presentation of metastasis was associated with a shorter median OS compared with bone-only disease (median 4.0, 3.1 and 6.4 years for mixed, visceral and bone-only metastasis, respectively; P = 0.0009; Fig. 3a). Moreover, the number of metastatic sites was also significantly related to OS (P < 0.0001; Fig. 3b). After diagnosis of metastatic disease, patients with 1–2 metastatic site involvements survived a median of 5.5 years, whereas those with more than or equal to three involved sites only survived a median of 2.2 years.
Fig. 3.
Kaplan–Meier estimates of overall survival by bone-only versus mixed versus visceral-only involvement (a); and by the number of metastatic sites at initial diagnosis: 1 versus 2 versus 3 metastatic sites (b)
In addition, we recalculated TTP and OS from the date of initial diagnosis of primary tumor to the time of locoregional recurrence or distant metastasis for those patients who presented at MDACC without metastatic disease (TTP); and OS from the time diagnosis of primary tumor to the time of death or the time of last contact. Median TTP = 2.71 years, 95 % CI = (2.36, 3.49) years and median OS = 9.18 years, 95 % CI = (6.57, 15.59) years. Specifically, compared to patients with stage I tumor at presentation, patients with stage II/III tumors had significantly shorter TTP (HR (95 %CI) = 2.255 (1.194–4.259), P = 0.0122 for III versus I, 1.853 (1.084–3.167), P = 0.0243 for II vs. I); patients with higher nuclear grade (grade = 3) had shorter TTP than those with lower nuclear grade (grade = 1,2) (HR (95 %CI) = 1.834 (1.116–3.013), P = 0.0167); patients with TN tumors had shorter TTP than ER/PR(+) patients (HR (95 %CI) = 2.696 (1.664–4.365), P ≤ 0.0001). In the multivariate analysis of OS, compared to patients with N0, 1 & 2 tumor at presentation, patients with N3 tumor had shorter OS (HR (95 %CI) = 4.192 (2.328–7.547), P < 0.0001); patients with higher nuclear grade (grade = 3) had shorter OS than those with lower nuclear grade (grade =1, 2) (HR (95 %CI) = 2.692 (1.357–5.341), P = 0.0046); patients with TN tumors had shorter OS than ER/PR(+) patients (HR (95 %CI) = 2.951 (1.753–4.966), P < 0.0001). No significant effects of BRCA positivity on time-to-event outcomes were detected (P > 0.55) in the multivariate models.
In addition, we calculated both the TTP and OS from the time of diagnosis of primary breast cancer to see if it would yield any differences in outcome. significant effects of BRCA positivity on time-to-event outcomes are detected (P-value >0.55) in the multivariate models.
Univariate and multivariate analyses
In univariate analyses for TTP, N3, nuclear grade 3, and TN tumors, and visceral involvement at initial presentation of metastasis, or involvement of ≥3 different metastatic sites were independent predictors for a shorter TTP. In contrast, patients with an initial M0 status and a family history of breast cancer had a longer TTP (Table 3). In multivariate analyses, patients with stage III disease (HR = 1.89; 95 % CI: 1.01–3.54; P = 0.001), visceral-only involvement (HR = 2.90; 95 % CI: 1.69–4.97; P = 0.003), or both visceral and bone involvement (HR = 3.66; 95 % CI: 2.16–6.21; P <0.0001) at initial presentation of metastasis had an increased risk of progression (Table 4). After adjusting for the significant variables, BRCA1 or BRCA2 status was not associated with TTP (HR = 0.83; 95 % CI: 0.47–1.49, and HR = 1.73; 95 % CI: 0.81–3.71; P = 0.28).
Table 3.
Univariate Cox proportional hazards model for time-to progression (TTP), and overall survival (OS)
| Time-to progression |
OS |
|||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |
| BRCA status | ||||||
| BRCA1/2 positive vs. negative | 1.34 | 0.90–2.02 | 0.15 | 1.48 | 0.89–2.44 | 0.13 |
| BRCA1 positive vs. negative | 1.43 | 0.89–2.28 | 0.30* | 2.2 | 1.28–3.78 | 0.004 |
| BRCA2 positive vs. negative | 1.19 | 0.60–2.35 | 0.52 | 0.16–1.67 | 0.27 | |
| Age | ||||||
| >38.6 vs. ≤38.6 | 0.92 | 0.66–1.28 | 0.62 | 0.92 | 0.61–1.40 | 0.72 |
| Tumor size of primary tumor | ||||||
| T4 vs. T1 | 1.73 | 1.01–2.97 | 0.20* | 1.97 | 0.93–4.14 | 0.08* |
| T3 vs. T1 | 1.56 | 0.83–2.91 | 2.46 | 1.19–5.09 | ||
| T2 vs. T1 | 1.27 | 0.83–1.93 | 1.65 | 0.93–2.92 | ||
| Nodal status | ||||||
| N3 vs. N0 | 1.69 | 1.00–2.86 | 0.03* | 2.15 | 1.16–3.97 | 0.01* |
| N2 vs. N0 | 0.78 | 0.44–1.38 | 1.03 | 0.52–2.05 | ||
| N1 vs. N0 | 0.80 | 0.52–1.22 | 0.76 | 0.43–1.33 | ||
| Clinical stagea | ||||||
| M0 vs. M1 | 0.45 | 0.28–0.73 | 0.001 | 0.47 | 0.25–0.90 | 0.02 |
| Nuclear grade | ||||||
| 3 vs. 1/2 | 1.93 | 1.19–3.11 | 0.006 | 2.38 | 1.38–4.10 | 0.001 |
| ER-status | ||||||
| Positive vs. negative | 0.83 | 0.59–1.17 | 0.30 | 0.54 | 0.35–0.83 | 0.004 |
| PR-status | ||||||
| Positive vs. negative | 0.73 | 0.52–1.04 | 0.08 | 0.46 | 0.29–0.74 | 0.001 |
| HER2-status | ||||||
| Positive vs. negative | 0.68 | 0.44–1.06 | 0.09 | 0.50 | 0.28–0.91 | 0.02 |
| Triple-negative | ||||||
| Yes vs. no | 1.78 | 1.18–2.68 | 0.005 | 2.60 | 1.62–4.14 | \0.0001 |
| FH of breast cancer | ||||||
| Positive vs. negative | 0.61 | 0.42–0.90 | 0.01 | 0.62 | 0.39–1.00 | 0.05 |
| FH of ovarian cancer | ||||||
| Positive vs. negative | 0.82 | 0.50–1.33 | 0.42 | 1.19 | 0.68–2.09 | 0.53 |
| Site of first metastasis | ||||||
| Visceral vs. bone-only | 2.16 | 1.40–3.33 | <0.0001* | 2.04 | 1.15–3.61 | 0.002* |
| Mixed vs. bone-only | 2.89 | 1.83–4.57 | 3.02 | 1.70–5.37 | ||
| Number of metastatic sites | ||||||
| 3 vs. 1–2 | 2.08 | 1.41–3.06 | 0.0002 | 2.75 | 1.77–4.27 | <0.0001 |
| Herceptin use | ||||||
| Yes vs. no | 0.95 | 0.63–1.42 | 0.81 | 0.60 | 0.33–1.06 | 0.08 |
| Lapatinib use | ||||||
| Yes vs. no | 1.26 | 0.80–1.99 | 0.31 | 0.90 | 0.50–1.63 | 0.74 |
| Bisphosphonates use | ||||||
| Yes vs. no | 0.79 | 0.55–1.12 | 0.19 | 0.61 | 0.38–0.97 | 0.04 |
P value for overall effect
Clinical stage at initial diagnosis
Table 4.
Multivariate Cox proportional hazards model for time-to progression (TTP), and overall survival (OS)
| Time-to progression |
OS |
|||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |
| BRCA status | ||||||
| BRCA1 positive vs. negative | 0.83 | 0.47–1.49 | 0.28 | 1.21 | 0.60–2.43 | 0.44 |
| BRCA2 positive vs. negative | 1.73 | 0.81–3.71 | 0.44 | 0.10–1.86 | ||
| Clinical stagea | ||||||
| IV vs. I | 0.57 | 0.30–1.08 | 0.001* | – | – | – |
| III vs. I | 1.89 | 1.01–3.54 | ||||
| II vs. I | 1.05 | 0.59–1.86 | ||||
| Site of first metastasis | ||||||
| Visceral vs. bone-only | 2.90 | 1.69–4.97 | 0.003 | – | – | – |
| Mixed vs. bone-only | 3.66 | 2.16––6.21 | <0.0001 | |||
| Nodal status | ||||||
| N3 vs. N0-2 | – | – | – | 2.69 | 1.49–4.88 | 0.0003 |
| Nuclear grade | ||||||
| 3 vs. 1/2 | – | – | – | 2.89 | 1.39–6.01 | 0.005 |
| Triple-negative | ||||||
| Yes vs. no | – | – | – | 2.15 | 1.19–3.90 | 0.001 |
| Bisphosphonates use | ||||||
| Yes vs. no | – | – | – | 0.55 | 0.30–0.99 | 0.03 |
HR hazard ratio, CI confidence interval
P value for overall effect
Clinical stage at initial diagnosis
In univariate analyses for OS, BRCA1 carriers, patients with N3 status, nuclear grade 3, and TN tumors, and patients with visceral involvement at initial presentation of metastasis, or involvement of ≥3 different metastatic sites had a shorter OS (Table 3). Additionally, positive family history of breast cancer, initial M0 status, ER/PR/HER2-positivity, and use of bisphosphonates were associated with a longer OS. Multivariate analysis showed that patients with N3 disease (HR = 2.69; 95 % CI: 1.49–4.88; P = 0.0003), nuclear grade 3 (HR = 2.89; 95 % CI: 1.39–6.01; P = 0.005) and TN tumors (HR = 2.15; 95 % CI: 1.19–3.90; P = 0.001) had an increased risk of death, and patients who had received bisphosphonates had a decreased risk of death (HR = 0.55; 95 % CI: 0.30–0.99; P = 0.04). Compared to non-carriers, BRCA1 carriers had shorter OS (HR = 1.21; 95 % CI: 0.60–2.43), while BRCA2 carriers had longer OS (HR = 0.44; 95 % CI: 0.10–1.86). However, the differences were not statistically significant (P = 0.44) after adjusting for all factors. In this paper, we also analyzed the TTR among patients who did not present with metastatic disease initially. In the univariate analysis for TTR, BRCA1/2 carriers had a higher risk of breast cancer recurrence compared to non-carriers (HR = 1.84; 95 % CI: 1.24–2.72; P = 0.0024). Compared to non-carriers, BRCA1 carriers had a higher risk of recurrence/distant metastases (HR = 3.23; 95 % CI: 2.06–5.10; P < 0.0001). In contrast, compared to non-carriers, BRCA2 carriers had a non-significantly lower risk of recurrence/distant metastases (HR = 0.80; 95 % CI: 0.40–1.65; P = 0.55). In multivariate analysis of TTR, higher clinical stage (II/III vs. I; P = 0.027), higher nuclear grade (3 vs. 1/2; P = 0.033) and positive TN-status (yes vs. no; P = 0.0001) remained significant for increased risk of breast cancer recurrence or distant metastases. However, no significant effects of BRCA positivity on TTR were detected (P >0.377) in the multivariate models.
Discussion
Our data indicate that BRCA1 carriers with MBC have shorter TTP and OS compared to non-carriers and BRCA2 carriers. Yet, in our multivariate analysis, BRCA status is not an independent adverse prognostic factor when other variables are considered. This could be due to the small number of BRCA carriers, especially of BRCA2 carriers. To the best of our knowledge, this is the largest study reported up to date evaluating the dissemination patterns, prognostic factors and outcomes in patients diagnosed with BRCA-associated and non-BRCA-associated MBC. We also observed that patients with TN breast cancer have worse outcomes with a median OS of 1.5 years, irrespective of their BRCA status confirming our previous observations [24, 25].
Consistent with the previous findings [26, 27], BRCA1 carriers were more likely to have high nuclear grade and TN tumors than non-carriers. On the other hand, the ER-positivity rate for BRCA1 carriers in this study (36.7 %) was higher compared with the previous published studies [28, 29]. However in both studies the median age at diagnosis for BRCA1 carriers were 42.3 and 38, respectively. In this study, the median age at diagnosis for BRCA1 carriers was 44.2 and this well may explain the high ER-positivity rate as suggested in a report by Zakhartseva et al. [30] that the ER-negative tumors were more frequently found in young women with BRCA1/2 mutations. Interestingly, the median age and the clinical stage at diagnosis were similar between the three groups.
In addition, the rates of BRCA-positivity between “white/black” versus “the others” (of which the majority were Latin-American) were inconsistent. However, the uncertainty regarding the prevalence estimates of deleterious mutations in non-White populations still exists and direct comparisons of mutation prevalence by ethnicity are few. Hall et al. [31] reported that women of African (15.6 %) and Latin American (14.8 %) ancestries had a significantly higher prevalence of deleterious BRCA1/2 mutations compared to women of Western European ancestry (12.1 %), primarily due to an increased prevalence of BRCA1 mutations in these two groups. However, a second clinic-based study of women with early onset (<50 years) breast cancer found similar rates of mutations among African American and white women (both 17 %) [32]. More recently, researchers from Northern California have published estimates of BRCA1 mutation prevalence in non-Jewish white (2.2 %), Hispanic (3.5 %), African American (1.3 %), and Asian American (0.5 %) women with breast cancer under age 65 years in a population-based study [33]. So, it has been unclear to what degree BRCA1/2 mutation frequency differs among women of diverse ethnicities. Herein, we identified 5 independent prognostic variables for survival; however unlike the previous studies, the number of sites of metastases was not associated with survival [30–34].
The family history association with longer TTP seems counterintuitive when the mutation carriers are likely to have stronger family history than non-carriers (depending of selection criteria for genetic testing). The reason behind this discrepancy could not be explained by the late presentation and therefore advanced stage of breast cancer diagnosis in women without any family history of breast cancer.
The median time from BRCA gene test to diagnosis of MBC of this study population is 25.5 months. We also compared the clinical and pathologic characteristics between patients who had their genetic counseling and testing within 12 months of their diagnosis (Group A) with the rest of the study populations (Group B). Except that Group B patients tended to have early stage (I/II) primary lesion than Group A patients, the two groups had similar characteristics. With regard the survival outcomes, the conclusions from the analysis of this subgroup remained the same as the ones obtained from the analyses of the whole study population (data not shown). However in our multivariate analysis of the effects of BRCA mutation status on survival outcomes (TTP, TTR and OS), we already adjusted for potential confounders of BRCA mutation status. Authors do not think that this subgroup analysis can avoid any selection bias considering that it is still a convenient sample, not a random sample.
Mouse models and pilot epidemiology studies have shown that inherited polymorphisms are associated with an inherited risk of tumor progression and poor outcome in human breast cancer [34–37]. Using an animal model, Lifsted et al. [35] demonstrated that the genetic background had a significant impact on its ability to form pulmonary metastases. Subsequently, systems genetics approaches have identified a number of polymorphic metastasis efficiency genes [36–39]. More recently, significant association of variants in RRP1B and SIPA1 with metastasis-free survival was observed, validating their role as inherited metastatic susceptibility genes [40]. Unexpectedly, this association with metastasis-free survival was restricted to ER-positive, lymph node-negative patients (P = 0.01). The lack of association in the LN+ samples supports the previous observations suggesting that breast cancer subtypes as defined by gene expression profiles [5] demonstrate preferential sites of relapse [8]. For example, women with TN breast cancer are less likely to experience a local recurrence before developing a distant recurrence [41]. Similarly, BRCA1 carriers, whose tumors express ER less frequently, have been shown to be less likely to have positive axillary LNs at diagnosis than non-hereditary breast cancers [42].
While the previous studies have shown that presence of lung metastases, ER-negativity, and basal-like tumors as major independent risk factors for brain metastases or visceral metastasis [41, 43–46], the data on the biology of organ-specific metastasis are currently scarce. Wang et al. [47] reported a 76-gene profile that can identify breast cancer patients at high risk for a distant metastasis within 5 years; however they were not able to correlate this high-risk profile to the site of relapse, nor did they find an association between the time until distant metastasis and site of relapse in their extensive patient database. Later on, Smid et al. [48] identified a panel of 69 differentially expressed gene profiles from primary tumors relapsing to bone compared to that of tumors relapsing elsewhere in the body. These results suggest that the aggressiveness of a tumor (ability to metastasize) is distinct from the tumor’s ability to home and proliferate in an organ-specific manner. Albiges et al. [49] showed that BRCA1 carriers had less bone (P = 0.01) and more lung (P = 0.005) metastases. Additionally, in the same study 10 of 15 (67 %) BRCA1 mutation carriers developed brain metastases, while no (0 %) BRCA2 carriers and six of 58 (10 %) non-carriers did (P = 10−5). In our cohort, tumors in BRCA carriers did not demonstrate a different constitutional predisposition to metastatic disease or a characteristic proclivity to metastasize to certain organs in an organ-specific manner. It is possible that inherited mutations of BRCA 1/2 genes might alter the susceptibilities of tumors to disseminate by activating different pathways; however, at present, neither of these genes have been directly implicated in the expression of extracellular matrix genes, angiogenesis or lymphangiogenesis promoting metastatic spread or virulence in an organ-specific manner [50].
There are little data on the efficacy of systemic therapy in BRCA1/2-associated MBC. Preclinical studies have shown that mouse and human cell lines without functional BRCA1/2 proteins have an increased sensitivity to agents causing double-strand DNA breaks, such as platinum and anthracyclines [51–54]. Likewise, Kriege et al. [55] observed an increased response rate (89 % vs. 50 %; P = 0.001), a longer TTP (HR = 0.64; P = 0.04) and a prolonged OS (HR = 0.53; P = 0.005) after start of first-line chemotherapy in BRCA2-associated MBC as compared to sporadic breast cancer patients. For BRCA1 mutation carriers compared to sporadic cases, a non-significant trend for an increased response rate (66 % vs. 50 %; P = 0.07) and a longer TTP (HR = 0.79; P = 0.14) were observed. Moreover, in women with MBC, BRCA1 was shown to be an independent predictor for a shorter TTP on taxane-based therapy while this effect was not observed for anthracycline-based chemotherapy [56, 57]. In our study cohort, BRCA1 carriers who mainly developed TN disease, relapsed quickly on palliative chemotherapy, with median durations of treatment in first-, second-, and third-line of only 16, 24, and 20 weeks, respectively. Yet, the fact that the outcomes were similar between BRCA1 and BRCA2 carriers and non-carriers, majority of whom had received anthracycline-taxane-containing chemotherapy regimens, suggests that BRCA mutant MBCs are just as sensitive to conventional chemotherapy regimens as other high grade breast cancers.
We also would like to emphasize that like their counterparts with locally advanced or early-stage invasive breast cancer, patients diagnosed with MBC warrant genetic risk assessment and testing on the basis of high-risk variables. Notably, the knowledge of a BRCA1/2 mutation is likely to significantly change the assessment of a patient’s or her family members’ risks for future cancers and the cancer prevention/risk reduction recommendations that would be considered. In addition, the identification of BRCA mutation carriers diagnosed with MBC will accelerate the conduct of future clinical trials which will help us identify their prognosis and also the chemosensitivity profile.
Certain limitations of this study should be acknowledged. Patients were selected from the total group of patients being referred for mutation testing to the MD Anderson, department of Breast Medical Oncology. Thus, the BRCA non-carrier control group may not be a fair representation of sporadic cancers for whom the usual course of MBC could be different. This may also have limited any differences seen between BRCA mutation carriers and non-carriers from the usual group of women with breast cancers. Future studies that prospectively test for BRCA mutations in women treated with systemic therapy should be conducted in order to eliminate the possibility of selection bias. The shorter median follow-up among BRCA1 carriers may have affected the survival outcomes after the diagnosis of MBC. Our sample size is small and we only identified 41 mutation carriers. Especially, the number of BRCA2 carriers is too small to show any meaningful statistical significance; however the clinical and disease characteristics seen more frequently in BRCA2 carriers supports the findings of our previous study [24]. In that study, BRCA2 carriers also tended to have a lower grade and ER(+) tumors compared to BRCA1 carriers. Herein, we did an exploratory analysis by excluding the BRCA2 carriers, and none of the statistical comparisons yielded any difference. Yet, we need to emphasize that although we did not see any clear survival differences or major differences in ethnicity, tumor size, HER2 status between the three groups, potentially important differences that are all associated with survival could have emerged if the size of the study cohort was larger.
In conclusion, our study showed that BRCA1 carriers with MBC have shorter TTP, and OS compared to non-carriers and BRCA2 carriers in this selected population of high-risk women with MBC. However, BRCA status was not an independent adverse prognostic factor when other variables were considered, which can be related to the small sample size. Furthermore, we demonstrate that patients with TN MBC carry a poorer prognosis irrespective of their BRCA status. Future randomized studies with larger prospective cohorts and longer-term follow-up are needed to validate these findings.
Acknowledgments
This work was supported by the Lynne Cohen Breast and Ovarian Cancer Project; Texas Business Women Funding; and the Nellie B. Connally Breast Cancer Research Fund, and the MD Anderson Cancer Center Support Grant 2P30 CA016672.
Footnotes
Conflict of interest Authors have no conflict interest to declare.
Contributor Information
S. Bayraktar, Division of Cancer Medicine, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA, soley.bayraktar@gmail.com.
A. M. Gutierrez-Barrera, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
H. Lin, Department of Biostatistics, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA
N. Elsayegh, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
T. Tasbas, Division of Cancer Medicine, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA; Department of Biostatistics, The University of Texas MD, Anderson Cancer Center, Houston, TX, USA.
J. K. Litton, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
N. K. Ibrahim, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
P. K. Morrow, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
M. Green, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
V. Valero, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
D. J. Booser, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
G. N. Hortobagyi, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
B. K. Arun, Department of Breast Medical Oncology, The University of Texas MD Anderson Cancer Center, 1515 Holcombe, Boulevard, Unit 1354, Houston, TX 77030-4009, USA
References
- 1.Ries LAG, Eisner MP, Kosary CL, Hankey BF, et al. [Accessed 26 Jan 2013];SEER cancer statistics review, 1975–2002. 2002 http://seer.cancer.gov/csr/1975_2002/
- 2.O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 10. 2005;(Suppl 3):20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 3.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12(1):20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Buzdar AU, Kau SWC, Hortobagyi GN. Improvement in breast cancer survival: results from M.D. Anderson Cancer Center protocols from 1975–2000. Proc Am Soc Clin Oncol; 2002 ASCO Annual Meeting; 2002. 2002 (abstr 212) [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smid M, Wang Y, Zhang Y, et al. Subtypes of breast cancer show preferential site of relapse. Cancer Res. 2008;68:3108–3114. doi: 10.1158/0008-5472.CAN-07-5644. [DOI] [PubMed] [Google Scholar]
- 9.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metas-tases. Cancer. 2008;113(10):2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao D, Du J, Cong L, et al. Risk factors for initial lung metastasis from breast invasive ductal carcinoma in stages I–III of operable patients. Jpn J Clin Oncol. 2009;39(2):97–104. doi: 10.1093/jjco/hyn133. [DOI] [PubMed] [Google Scholar]
- 11.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52(1):108–118. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy RD, Quinn J, Johnston PH, Harkin DP. BRCA1: mechanisms of inactivation and implications for management of patients. Lancet. 2002;360:1007–1014. doi: 10.1016/S0140-6736(02)11087-7. [DOI] [PubMed] [Google Scholar]
- 13.Verhoog LC, Brekelmans CT, Seynaeve C, et al. Survival in hereditary breast cancer associated with germline mutations of BRCA2. J Clin Oncol. 1999;17:3396–3402. doi: 10.1200/JCO.1999.17.11.3396. [DOI] [PubMed] [Google Scholar]
- 14.Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357(2):115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 15.Brekelmans CT, Tilanus-Linhorst MM, Seynaeve C, et al. Tumour characteristics, survival and prognostic factors of hereditary breast cancer from BRCA2-, B. Eur J Cancer. 2007;43:867–876. doi: 10.1016/j.ejca.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Distant disease-free interval, site of first relapse and post-relapse survival in BRCA carriers. Breast Cancer Res Treat. 2008;111:303–311. doi: 10.1007/s10549-007-9781-7. [DOI] [PubMed] [Google Scholar]
- 17.Bonadona V, Dussart-Moser S, Voirin N, et al. Prognosis of early-onset breast cancer based on BRCA1/2 mutation status in a French population-based cohort and review. Breast Cancer Res Treat. 2007;101(2):233–245. doi: 10.1007/s10549-006-9288-7. [DOI] [PubMed] [Google Scholar]
- 18.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010;119(1):13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 19.Tan DS, Rothermundt C, Thomas K, et al. “BRCAness” syndrome in ovarian cancer: a case-control study describing the clinical features and outcome of patients with epithelial ovarian cancer associated with BRCA1 and BRCA2 mutations. J Clin Oncol. 2008;26(34):5530–5536. doi: 10.1200/JCO.2008.16.1703. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. JAMA. 2011;306(14):1557–1565. doi: 10.1001/jama.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alsop K, Fereday S, Meldrum C, et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol. 2012;30(21):2654–2663. doi: 10.1200/JCO.2011.39.8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singletary SE, Greene FL. Revision of breast cancer staging: the 6th edition of the TNM Classification. Semin Surg Oncol. 2003;21(1):53–59. doi: 10.1002/ssu.10021. [DOI] [PubMed] [Google Scholar]
- 23.Black MM, Speer FD. Nuclear structure in cancer tissues. Surg Gynecol Obstet. 1957;105(1):97–102. [PubMed] [Google Scholar]
- 24.Bayraktar S, Gutieerz-Barrera A, Liu DD, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treatment. 2011;130(1):145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayraktar S, Gutierrez-Barrera AM, Liu D, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130(1):145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakhani SR, Jacquemier J, Sloane JP, et al. Multifactorial analysis of differences between sporadic breast cancers and cancers involving BRCA1 and BRCA2 mutations. J Natl Cancer Inst. 1998;90(15):1138–1145. doi: 10.1093/jnci/90.15.1138. [DOI] [PubMed] [Google Scholar]
- 27.Atchley DP, Albarracin CT, Lopez A, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26(26):4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrski T, Gronwald J, Huzarski T, et al. Response to neo-adjuvant chemotherapy in women with BRCA1-positive breast cancers. Breast Cancer Res Treat. 2008;108(2):289–296. doi: 10.1007/s10549-007-9600-1. [DOI] [PubMed] [Google Scholar]
- 29.Arun B, Bayraktar S, Liu DD, et al. Response to neoad-juvant systemic therapy for breast cancer in BRCA mutation carriers and noncarriers: a single-institution experience. J Clin Oncol. 2011;29(28):3739–3746. doi: 10.1200/JCO.2011.35.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakhartseva LM, Gorovenko NG, Podolskaya SV, et al. Breast cancer immunohistochemical features in young women with BRCA 1/2 mutations. Exp Oncol. 2009;31(3):174–178. [PubMed] [Google Scholar]
- 31.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haffty BG, Silber A, Matloff E, et al. Racial differences in the incidence of BRCA1 and BRCA2 mutations in a cohort of early onset breast cancer patients: African American compared to white women. J Med Genet. 2006;43(2):133–137. doi: 10.1136/jmg.2005.034744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John EM, Miron A, Gong G, et al. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298(24):2869–2876. doi: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 34.Hunter KW, Broman KW, Voyer TL, et al. Predisposition to efficient mammary tumor metastatic progression is linked to the breast cancer metastasis suppressor gene Brms1. Cancer Res. 2001;61(24):8866–8872. [PubMed] [Google Scholar]
- 35.Lifsted T, Le Voyer T, Williams M, et al. Identification of inbred mouse strains harboring genetic modifiers of mammary tumor age of onset and metastatic progression. Int J Cancer. 1998;77(4):640–644. doi: 10.1002/(sici)1097-0215(19980812)77:4<640::aid-ijc26>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 36.Park YG, Zhao X, Lesueur F, et al. Sipa1 is a candidate for underlying the metastasis efficiency modifier locus Mtes1. Nat Genet. 2005;37(10):1055–1062. doi: 10.1038/ng1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crawford NP, Qian X, Ziogas A, et al. Rrp1b, a new candidate susceptibility gene for breast cancer progression and metastasis. PLoS Genet. 2007;3(11):e214. doi: 10.1371/journal.pgen.0030214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crawford NP, Alsarraj J, Lukes L, et al. Bromodomain 4 activation predicts breast cancer survival. Proc Natl Acad Sci USA. 2008;105(17):6380–6385. doi: 10.1073/pnas.0710331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford NP, Walker RC, Lukes L, et al. The Diasporin Pathway: a tumor progression-related transcriptional network that predicts breast cancer survival. Clin Exp Metastasis. 2008;25(4):357–369. doi: 10.1007/s10585-008-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh SM, Look MP, Sieuwerts AM, et al. Distinct inherited metastasis susceptibility exists for different breast cancer subtypes: a prognosis study. Breast Cancer Res. 2009;11(5):R75. doi: 10.1186/bcr2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 42.Foulkes WD, Metcalfe K, Hanna W, et al. Disruption of the expected positive correlation between breast tumor size and lymph node status in BRCA1-related breast carcinoma. Cancer. 2003;98(8):1569–1577. doi: 10.1002/cncr.11688. [DOI] [PubMed] [Google Scholar]
- 43.Liedtke C, Mazouni C, Hess KR, et al. Response to neo-adjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008;26(8):1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Pinilla SM, Sarrio D, Honrado E, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006;12(5):1533–1539. doi: 10.1158/1078-0432.CCR-05-2281. [DOI] [PubMed] [Google Scholar]
- 45.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 46.Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101(4):810–816. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 48.Smid M, Wang Y, Klijn JG, et al. Genes associated with breast cancer metastatic to bone. J Clin Oncol. 2006;24(15):2261–2267. doi: 10.1200/JCO.2005.03.8802. [DOI] [PubMed] [Google Scholar]
- 49.Albiges L, Andre F, Balleyguier C, et al. Spectrum of breast cancer metastasis in BRCA1 mutation carriers: highly increased incidence of brain metastases. Ann Oncol. 2005;16(11):1846–1847. doi: 10.1093/annonc/mdi351. [DOI] [PubMed] [Google Scholar]
- 50.Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–2823. doi: 10.1056/NEJMra0805239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kennedy RD, Quinn JE, Mullan PB, et al. The role of BRCA1 in the cellular response to chemotherapy. J Natl Cancer Inst. 2004;96(22):1659–1668. doi: 10.1093/jnci/djh312. [DOI] [PubMed] [Google Scholar]
- 52.Foulkes WD. BRCA1 and BRCA2: chemosensitivity, treatment outcomes and prognosis. Fam Cancer. 2006;5(2):135–142. doi: 10.1007/s10689-005-2832-5. [DOI] [PubMed] [Google Scholar]
- 53.James CR, Quinn JE, Mullan PB, et al. BRCA1, a potential predictive biomarker in the treatment of breast cancer. Oncologist. 2007;12(2):142–150. doi: 10.1634/theoncologist.12-2-142. [DOI] [PubMed] [Google Scholar]
- 54.Robson ME, Chappuis PO, Satagopan J, et al. A combined analysis of outcome following breast cancer: differences in survival based on BRCA1/BRCA2 mutation status and administration of adjuvant treatment. Breast Cancer Res. 2004;6(1):R8–R17. doi: 10.1186/bcr658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kriege M, Seynaeve C, Meijers-Heijboer H, et al. Sensitivity to first-line chemotherapy for metastatic breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(23):3764–3771. doi: 10.1200/JCO.2008.19.9067. [DOI] [PubMed] [Google Scholar]
- 56.Kurebayashi J, Yamamoto Y, Kurosumi M, et al. Loss of BRCA1 expression may predict shorter time-to-progression in metastatic breast cancer patients treated with taxanes. Anticancer Res. 2006;26(1B):695–701. [PubMed] [Google Scholar]
- 57.Wysocki PJ, Korski K, Lamperska K, et al. Primary resistance to docetaxel-based chemotherapy in metastatic breast cancer patients correlates with a high frequency of BRCA1 mutations. Med Sci Monit. 2008;14(7):SC7–SC10. [PubMed] [Google Scholar]