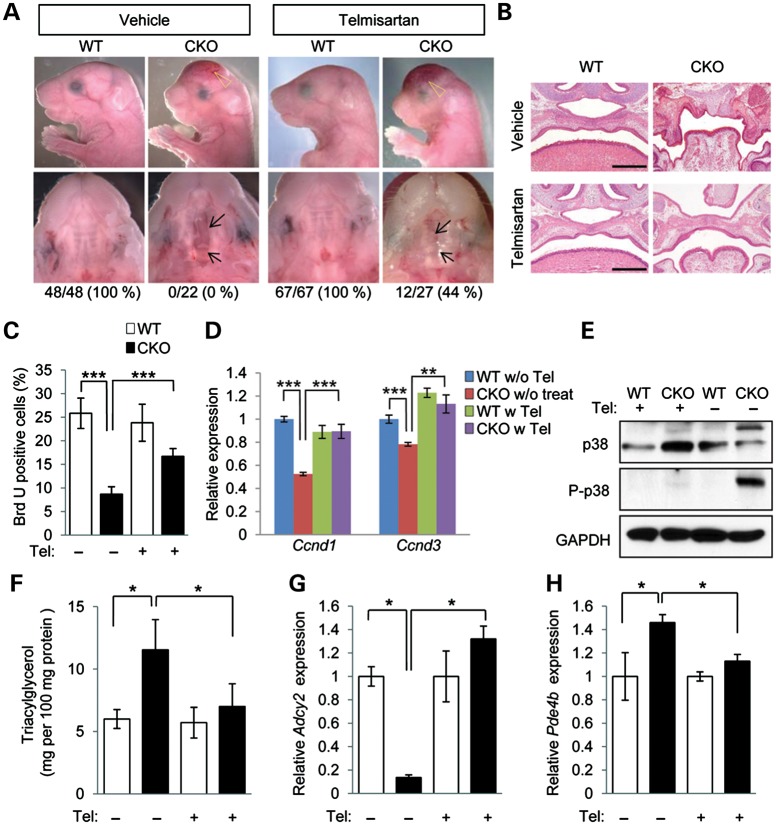
Figure 6.
Prevention of cleft palate in Tgfbr2 mutant embryos by telmisartan. (A) Lateral, frontal and palatal views of Tgfbr2fl/fl littermate (WT) and Tgfbr2fl/fl;Wnt1-Cre (CKO) mice after treatment with vehicle or telmisartan. Arrows indicate the edge of the palate. Open arrowheads show defects in the frontal bone. Appearance of the palate was scored as normal or cleft (below). (B) Histological observation of WT and CKO mice after treatment with vehicle or telmisartan. Lower panels show higher magnification of upper panels. Bars, 500 μm (upper panels) and 200 μm (lower panels). (C) Quantification of BrdU-positive cells in E14.5 palate of Tgfbr2fl/fl (WT) and Tgfbr2fl/fl;Wnt1-Cre (CKO) mice with or without telmisartan treatment. White bars, WT; black bars, CKO. Three samples per group were used. ***P < 0.001. (D) Quantitative RT–PCR analyses of Ccnd1 and Ccnd3 in WT and CKO MEPM cells with (w) or without (w/o) telmisartan (Tel) treatment. **P < 0.01, ***P < 0.001. (E) Immunoblotting analysis of p38 and phosphorylated p38 (P-p38) in WT and CKO mice after treatment with vehicle (−) or telmisartan (+). (F) Triacylglycerol level in E18.5 WT (white bars) and CKO (black bars) mice after treatment with telmisartan (+) or vehicle (−). *P < 0.05. (G and H) Quantitative RT–PCR analyses of Adcy2 (E) and Pde4b (F) in E14.5 WT and CKO palates. *P < 0.05.