Abstract
Mucin-type O-glycans are the primary constituents of mucins that are expressed on various mucosal sites of the body, especially the bacteria-laden intestinal tract. Mucins are the main components of mucus, which is secreted by goblet cells and forms a protective homeostatic barrier between the resident microbiota and the underlying immune cells in the colon. However, the specific role of mucin-type O-glycans in mucus barrier function has been uncertain. Recent studies utilizing mice deficient in key glycosyltransferases involved in O-glycan biosynthesis on intestinal mucins have underscored the importance of mucin-type O-glycosylation in mucus barrier function. This review will highlight recent advances in our understanding of mucin-type O-glycan function in the mucus barrier and how they promote mutualism with our resident microbiota.
Keywords: intestinal homeostasis, microbiota, mucin, mucin-type O-glycans, colitis
Introduction
Glycosylation is an essential mode of posttranslational modification of proteins. This is a complex process mediated by numerous and diverse enzymes, which catalyze the covalent addition of monosaccharides (called glycans) to specific amino acids found within target proteins (Spiro 2002). O-linked glycosylation is a major form of glycosylation characterized by the covalent addition of glycans to the hydroxyl (–OH) group of serine and threonine residues, creating the O-linkage in the initiating glycosylation event (Brockhausen et al. 2009). While there are several glycans that can be O-linked to Ser/Thr residues, including mannose, xylose, N-acetylglucosamine (GlcNAc) and others, O-linked N-acetylgalactosamine (O-GalNAc) glycans are of special relevance to mucosal sites such as the airways, urogenital and gastrointestinal tracts. These mucosal tissues highly express mucins, which are rich in Ser/Thr residues that are primary targets for O-glycosylation with GalNAc, creating the foundation upon which long and more complex oligosaccharide chains are built. O-glycans extend out from the mucin protein core and are intimately associated with the external environment. As such, mounting evidence has demonstrated that mucin-type O-glycans are pivotal in determining whether host diseases will be averted or promoted, with particular regard to interactions with microorganisms present in the environment. In this review, we will focus on the role of mucin-type O-glycans in promoting mutualism with the commensal microbiota in the intestinal tract.
The intestinal mucus layer and mucins
The intestinal mucus layer
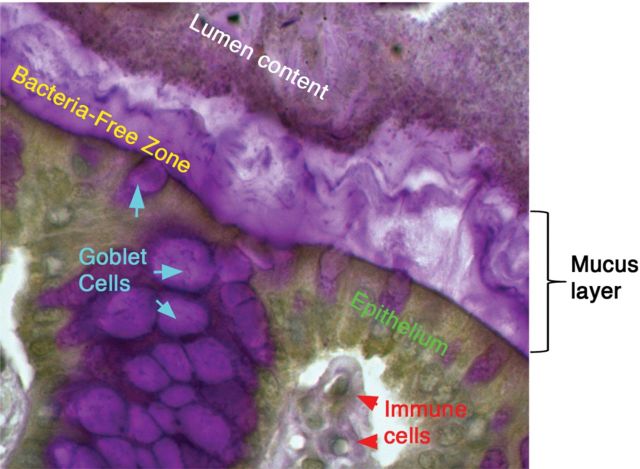
The intestinal tract is unique among mucosal tissues, because it is heavily colonized by microorganisms, mostly bacteria, starting from birth. In the colon, bacterial density is the highest, reaching up to 1 × 1012/g of lumen content (Sartor 2008). Recent work has established that the mucin-rich mucus layer acts as an essential barrier between the luminal microbiota and the underlying immune cells (Johansson et al. 2008) (Figure 1). While this layer is thin and discontinuous in the small intestine, it increases in thickness along the length of the colon (Johansson et al. 2011). The colonic mucus layer exhibits structural complexity by containing two sub-layers: An inner layer, which is 50–100 μm thick and firmly attached to the epithelium, and an outer layer, which is up to 800 μm thick and derived from and loosely attached to the inner layer (Atuma et al. 2001; Johansson et al. 2008). Intriguingly, these two layers mediate opposite interactions with the microbiota; whereas the outer layer is densely colonized by intestinal microbes, the inner layer is virtually devoid of bacteria, leaving a bacteria-free zone adjacent to the epithelia, thus demonstrating mucus as a critical mediator of host–bacteria interactions (Johansson, Holmen Larsson et al. 2011; Swidsinski et al. 2007). The physiological importance of mucus layer has been suggested for many years by studies in human ulcerative colitis (UC) patients, which have reported several defects in its function. These include decreased mucus layer thickness and increased penetration of mucus barrier by bacteria (Swidsinski et al. 2007), reduced synthesis and secretion of mucins (Tytgat et al. 1996) and altered O-glycosylation (Podolsky and Fournier 1988). While these studies reveal a link between defects in the intestinal mucus and UC, whether they reflect a primary or secondary event in disease pathogenesis is unclear. However, murine models of inflammatory bowel disease (IBD) have reported a defect in mucin biosynthesis prior to disease development (Schwerbrock et al. 2004). Mice deficient in Muc2, the principal mucin forming the intestinal mucus layers (Johansson et al. 2008) (discussed below), completely lack a colonic mucus layer and fail to restrict bacterial attachment to the mucosal tissues, which likely leads to the severe spontaneous colitis and colorectal cancer that these mice develop (Velcich et al. 2002; Van der Sluis et al. 2006). These studies provide a solid foundation supporting the importance of mucin function in the intestinal tract.
Fig. 1.
The intestinal mucus layer and host–microbiota interactions. Periodic acid-Schiff staining of the murine colon revealing the stratified mucus layer creating the bacteria-free zone between the bacteria-laden intestinal lumen and the underlying tissue. The mucus layer is primarily composed of Muc2 (human, MUC2), the major secreted gel-forming mucin of the intestinal tract. Muc2 is synthesized by goblet cells and is secreted as a polymer into the intestinal lumen where it coats the epithelial surface. In the colon, this layer is critical to separating the dense luminal content, including bacteria, from the underlying epithelial and immune cells to limit unwanted stimulation of the mucosal immune system. As described in the text, the Muc2-mucus layer is composed primarily of O-glycans.
Mucin family molecules
As alluded to above, the intestinal mucus is primarily composed of Mucin-2 (MUC2), a prominent member of the mucin family. Mucins represent a major group of high-molecular-weight O-linked glycoproteins, which are widely expressed in the body and play many essential roles in physiologic and pathologic settings (reviewed in Bafna et al. 2010; McGuckin et al. 2011). There are currently 20 known mucin genes in humans (Brockhausen et al. 2009), many of which are expressed in the gastrointestinal tract and can be broadly classified into three categories: secretory gel forming (MUC2, MUC5AC, MUC5B, MUC6), secretory nongel-forming (MUC7) and membrane-bound (e.g., MUC1, MUC3, MUC4, MUC12, MUC13, MUC17) (Lievin-Le Moal and Servin 2006). These categories reflect the diverse functions among different family members; however, what unifies them are the presence of large domains enriched with serine and threonine and also proline (called proline, threonine, serine-rich, or “PTS”, domains). (Johansson et al. 2011; Backstrom et al. 2013). In several MUC family members, especially gel-forming mucins, these Ser/Thr and Pro residues can be found in repeated sequences (Allen et al. 1998). As a result, these regions are extensively modified by O-glycosylation to create a highly heterogeneous array of oligosaccharides that confer upon mucins their individual functions.
In the intestine, MUC2 is the major secretory gel-forming mucin (Johansson et al. 2008) and is constitutively expressed by specialized epithelial cells called goblet cells (van Klinken et al. 1999). The biosynthesis of MUC2 is a complex, highly regulated process (Johansson et al. 2011, 2013; Backstrom et al. 2013). Once the MUC2 apoprotein core (∼5200 amino acids in size) is translated, MUC2 monomers form dimers though intermolecular disulfide bridging occurring between cysteine-knot domains located near the C-terminus (Asker et al. 1998). MUC2 dimers then traffic to the Golgi apparatus, where O-linked glycosylation takes place via the action of numerous glycosyltransferases (Axelsson et al. 2001; Johansson et al. 2011; Backstrom et al. 2013).
Biosynthesis of mucin-type O-glycans
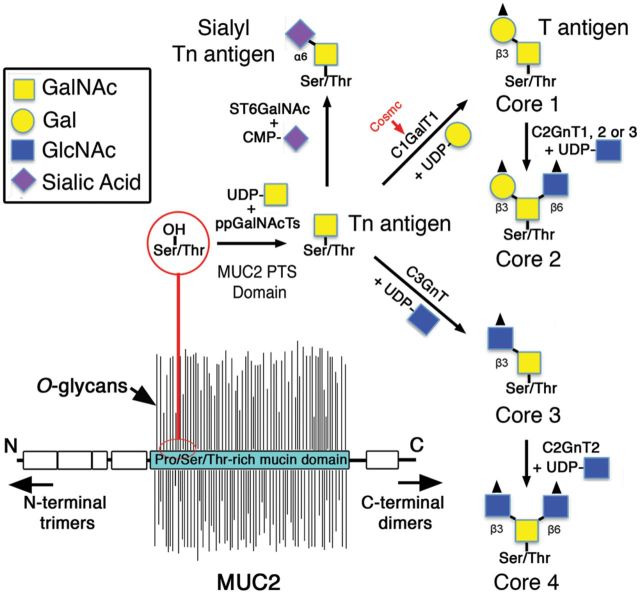
The first step in mucin-type O-glycosylation is the addition of N-acetylgalactosamine (GalNAc) from uridine diphospho (UDP)-GalNAc to Ser/Thr residues of the MUC2 PTS domains (Bennett et al. 2012). This reaction is catalyzed by a specific class of glycosyltransferases called polypeptidyl GalNAc transferases (ppGalNAcTs), of which there are at least 20 members in humans (Bennett et al. 2012). This GalNAcα-Ser/Thr structure forms the Tn antigen, which is the substrate for glycosyltransferases that give rise to core 1 and core 3 structures from which most mucin-type O-glycans are ultimately derived (Brockhausen et al. 2009) (Figure 2). The biosynthesis of core 1 O-glycans is initiated by a single gene encoding the enzyme core 1 β1,3-galactosyltransferase (C1GalT1, or T-synthase), which transfers galactose (Gal) from UDP-Gal to GalNAcα-O-Ser/Thr via a β1 → 3 linkage to the C3 carbon, forming the core 1 structure Galβ1 → 3GalNAcα-O-Ser/Thr (also known as T-antigen) (Fu et al. 2011; Ju et al. 2002). C1GalT1 is widely expressed in most tissues and cell types, including epithelial, endothelial and hematopoietic cells (Fu et al. 2011). The function of C1GalT1 requires the endoplasmic reticulum (ER) chaperone known as Cosmc (Ju and Cummings 2002), which inhibits aggregation and promotes stability of newly translated C1GalT1 (Wang et al. 2010). The T-antigen forms the basis for core 1-derived O-glycans, which are the major O-glycans found on mouse Muc2 (Fu et al. 2011; Thomsson et al. 2012).
Fig. 2.
The biosynthesis of mucin-type O-glycans. MUC2 is densely O-glycosylated within its PTS domains, giving the molecule its “bottle-brush” appearance. MUC2 O-glycosylation begins with the addition of GalNAc by the action of ppGalNAcTs, creating the Tn antigen. Tn antigen is modified by C1GalT1 via the help of the chaperone Cosmc to create the core 1 structure, or by C3GnT to create the core 3 structure, or else by ST6GalNAc (α2,6 sialytransferase) to form the sialyl Tn antigen. Core 1 and 3 structures can be extended to form the core 2 and 4 structures, respectively; however, sialyl Tn cannot be extended further. Moreover, each core structure can be further modified and/or terminated by fucosyltransferases, sulfotransferases or sialotransferases (not shown).
Core 2 O-glycans are derived from core 1 O-glycans and are thus an important component of the MUC2 O-glycome (Figure 2). The biosynthesis of core 2 O-glycans is mediated by the core 2 β1 → 6 N-acetylglucosaminyltransferases (C2GnTs), which catalyze the transfer of GlcNAc from UDP-GlcNAc to the C6 carbon of the initial GalNAc of the core 1 structure, forming the core 2 structure GlcNAcβ1-6(Galβ1-3)GalNAcα-Ser/Thr (Bierhuizen and Fukuda 1992; Brockhausen et al. 2009). C2GnTs have four members: C2GnT1, C2GnT2, C2GnT3 and I β1,6-N-acetylglucosaminyltransferase (IGNT) (Brockhausen et al. 2009). C2GnT1 and 3 are expressed widely by both hematopoietic cells and nonhematopoietic cells including gut epithelial cells (Stone et al. 2010). C2GnT1 and 3 are also designated C2GnT-L (leukocyte-type) due to their role in adding GlcNAcβ1-6 branches to mucin-like glycoproteins on high endothelial venules (HEV), building the sialyl Lewisx epitope that is recognized by lymphocyte selectins when homing to lymph nodes (Hemmerich et al. 1995; Schwientek et al. 2000). In contrast, C2GnT2 is known as C2GnT-M (mucus type), since it is highly restricted to mucin-producing tissues such as the gastrointestinal tract and airways (Yeh et al. 1999; Stone et al. 2010). Additionally, C2GnT2 can synthesize core 4 structures (the latter discussed below) as well as the branched I antigens (Yeh et al. 1999). IGNT, also called I-branching enzyme, cannot form core 2 structures but like C2GnT2 can add GlcNAcβ1-6 branches to Gal residues in polylactosamine chains, creating branch points for these oligosaccharides (Magnet and Fukuda 1997).
Unlike core 1 and 2 structures, core 3-derived O-glycans have a highly restricted expression pattern, as they are found primarily in the intestinal tract and the salivary glands (An et al. 2007; Brockhausen 2007; Brockhausen et al. 2009). The biosynthesis of core 3-derived O-glycans is catalyzed by the enzyme core 3 β1,3 N-acetylglucosaminyltransferase (C3GnT), which uses UDP-GlcNAc to add GlcNAc to the Tn antigen, forming the core 3 structure GlcNAcβ1 → 3GalNAcα-Ser/Thr (Iwai et al. 2002; An et al. 2007) (Figure 2). As noted above, the core 3 structure also forms the substrate for C2GnT2, leading to the synthesis of branched core 4 structure GlcNAcβ1 → 3(GlcNAcβ1 → 6)GalNAcαSer/Thr. Interestingly, in humans, core 3-derived structures are the major O-glycans found on MUC2 (Robbe et al. 2004; Thomsson et al. 2012).
O-glycans may also be modified by numerous additional glycosyltransferases and sulfotransferases that catalyze the addition of residues such as sulfate, sialic acid and fucose, creating biologically important structures (e.g., sialyl Lewisx or sulfo-sialyl Lewisx) (Brockhausen et al. 2009).
Mucin-type O-glycans extend out perpendicularly from the MUC2 protein core, giving the molecule its bottle brush-like appearance (Backstrom et al. 2013) (Figure 2). Collectively, O-glycans account for up to 80% of the total molecular mass of Muc2 (Johansson et al. 2011; Thomsson et al. 2011). The monomeric fully glycosylated MUC2 is ∼2.5 MDa, but the polymerized form can be >100 MDa (Johansson et al. 2011). Evidence strongly suggests that MUC2 polymerization occurs via the formation of N-terminal trimers of dimeric MUC2, leading to its enormous size (Godl et al. 2002; Johansson et al. 2011). Ultimately, polymeric MUC2 is released from the Golgi and packaged into secretory granules that are eventually released into the intestinal lumen to form the mucus layer. As noted above, mucus layer establishment is essential for intestinal homeostasis. However, the specific role of mucin-type O-glycans in mucus barrier function has remained enigmatic until recently. The following section will explore the most current advances in elucidating the contribution of mucin-type O-glycans to the intestinal barrier.
The roles of mucin-type O-glycans in intestinal barrier function
The role of core 1-derived O-glycans in intestinal barrier function
Core 1-derived O-glycans are the principal O-glycans expressed in most tissues (Byrd and Bresalier 2004). To evaluate their role specifically in the intestinal epithelium, we recently generated mice conditionally lacking C1GalT1 specifically in intestinal epithelial cells (IEC C1galt1−/− mice) (Fu et al. 2011). We found that loss of C1GalT1 efficiently removed all core 1-derived O-glycans and exposed the Tn antigen only in IEC and secreted Muc2 (Fu et al. 2011). Critically, we found that loss of core 1-derived O-glycans led to a rapid induction of severe spontaneous colitis by 2 weeks after birth, and the disease severity progressed over time (Fu et al. 2011) (Figure 3). Early infiltration of myeloid cells and formation of crypt abscesses especially in the distal colon and the rectum characterized the colitis, which bears a stark resemblance to the disease pattern observed in human UC (Fu et al. 2011). To explore the mechanism, we found that loss of core 1-derived O-glycans severely impaired establishment of the mucus layer in the murine colon. The impaired mucus barrier was characterized by dramatic thinning of the inner mucus layer and breaches in its structure compared with wild-type (WT) mice (Fu et al. 2011) (Figure 3). These mucus defects were also closely correlated with defective barrier function of the mucosa and greater translocation of bacteria into mucosal tissues (Fu et al. 2011). Recent studies have confirmed that the inner layer is more easily penetrated in IEC C1galt1−/− mice (Johansson, Gustafsson et al. 2010; Johansson et al. 2013). The colitis severity was dependent upon the microbiota, as depletion of commensal bacteria by treatment with broad-spectrum antibiotics ameliorated disease (Fu et al. 2011).
Fig. 3.
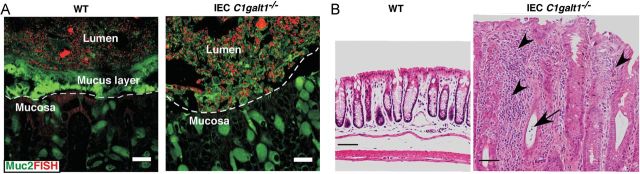
Loss of intestinal core 1-derived O-glycans impairs mucus barrier function and leads to spontaneous colitis. (A) Fluorescence in situ hybridization (FISH, bacterial 16S rDNA probe) revealed that WT colon epithelium was covered by an intact inner layer of mucus (FITC-labeled glycosylation-independent polyclonal antibody to Muc2) that was devoid of bacteria. In contrast, IEC C1galt1–/– epithelium had no mucus layer and directly contacted commensal bacteria. Dashed lines mark epithelial surfaces. Scale bar, 20 mm. (B) H&E staining showing distal colon sections from 16-week-old WT and IEC C1galt1−/− mice. Severe inflammatory disease that resembles human UC occurs in IEC C1galt1−/− mice, as shown by numerous infiltrating inflammatory cells (arrowheads), and a crypt abscess (arrow).
Because disease in IEC C1galt1−/− mice closely models human UC, we attempted to delineate the cellular mediators and possible innate pathways involved in disease, which are still unresolved questions in the current understanding of UC pathogenesis. Interestingly, we found that disease development was independent of T- and B-lymphocytes, as IEC C1galt1−/− mice on a Rag1-deficient background developed robust disease. However, disease severity did improve with age in these mice, showing that adaptive immunity is important for colitis progression (Fu et al. 2011). While the disease was dependent upon bacteria, we found that it was independent of signaling by key innate pathways mediated by the pattern recognition receptor Toll-like receptor (TLR) 4 and the major TLR family adaptor protein MyD88, since IEC C1galt1−/− mice deficient in either of these molecules still developed robust disease (Fu et al. 2011). Therefore, the innate immune pathways mediating the bacterial-induced colitis in the absence of intestinal core 1-derived O-glycans are still undefined. Interestingly, although C1GalT1 was also deleted in the small intestine in IEC C1galt1−/− mice, we have not observed any significant morphological changes or disease development in this region. This may likely be due to the more rapid transit and fewer levels of microbes that inhabit this region (Sartor 2008) as well as the increased production of antimicrobial genes by Paneth cells, which are normally only present in the small bowel (Ouellette 2010).
Our data further demonstrated that a subset of UC patients exhibited positive Tn antigen staining in epithelial cells. Interestingly, in a limited sample study, somatic missense mutations in the Cosmc gene were found in Tn-positive epithelial cells (Fu et al. 2011). This pilot study suggests a role for defective core 1 O-glycosylation in UC development in a subpopulation of UC patients, although a more rigorous study with additional patient samples is required to validate the result and improve its statistical power. However, this hypothesis was supported using mice containing a tamoxifen (TM)-inducible deletion of epithelial C1GalT1 (TM-IEC C1galt1−/−), which phenotypically recapitulates the effect of somatic loss-of-function mutations in Cosmc by abolishing synthesis of core 1-derived O-glycans in adult mice (Fu et al. 2011). TM-IEC C1galt1−/− mice show a rapid decrease in mucus layer thickness and the bacteria-free zone and increased bacterial translocation, and develop bacterial-dependent spontaneous colitis 2 weeks after TM treatment (Fu et al. 2011). Importantly, mucus depletion preceded the fulminate colitis development, supporting the etiological role for defective core 1 O-glycosylation and mucus barrier function in the onset of IBD. Indeed, similar defects in mucus layer function were recently demonstrated in human UC tissues (Johansson, Gustafsson et al. 2010). However, the question remains exactly how O-glycans promote mucus barrier integrity and homeostasis with the gut microbiota.
The importance of core 1-derived O-glycans is further underscored with the study of mice lacking the core 2 O-glycans. It was recently demonstrated that C2GnT2 was highly expressed in the small bowel and colon of mice (Stone et al. 2009). C2GnT2 deficiency affected mucin biochemical composition by reducing levels of core 2 and 4 O-glycans, as well as I-branching as expected (Stone et al. 2009). Interestingly, while these mice were not reported to develop spontaneous disease, they did have a baseline defect in intestinal permeability as measured by the Fluorescein isothiocyanate (FITC)-dextran assay (Stone et al. 2009). In addition, C2GnT2−/− mice were more susceptible to intestinal challenge using the cytotoxic agent dextran sodium sulfate (DSS), a common model to study acute colitis (Stone et al. 2009). Unfortunately, the impact of core 2/4 O-glycan deficiency on the mucus barrier and host–microbiota interactions was not addressed in this study (Stone et al. 2009). Further work is necessary to clarify the contribution of C2GnT2 to mucus quality and host–microbe homeostasis.
The role of core 3-derived O-glycans in promoting intestinal mucus barrier function
Core 3-derived O-glycans are important components of human colonic mucin-type O-glycans (Robbe et al. 2004). We addressed the role of core 3-derived O-glycans by first cloning murine C3GnT, which we showed bears 68% identity to human C3GnT (An et al. 2007). Like C1GalT1 for core 1 O-glycans, C3GnT in humans is the sole enzyme catalyzing the biosynthesis of core 3-derived O-glycans (An et al. 2007). Similar to humans, gene expression analysis and LacZ reporter gene strategies identified that C3GnT expression was highly restricted to the colon and, to a lesser extent, the small intestine and the salivary glands (An et al. 2007). We then generated C3GnT−/− mice, which led to a compete ablation of all core 3-derived O-glycans on colonic mucins but a retention of core 1- and 2-derived O-glycans as analyzed by Matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) (An et al. 2007). This has been confirmed in a recent study (Thomsson et al. 2012) and indicates that, like humans, murine C3GnT is likely the only enzyme responsible for synthesis of the core 3 structure in mice. Further characterization of C3GnT−/− mice revealed that loss of core 3-derived O-glycans led to a reduction in Muc2 protein expression in goblet cells and partial exposure of the Tn antigen compared with WT mice (An et al. 2007). Although we did not observe any spontaneous disease, we found that loss of core 3-derived O-glycans led to a number of physiological defects, including increases in intestinal permeability and higher levels of colonic bacteria within the colonic mucosa (An et al. 2007). No differences were seen in the total levels of colonic bacteria addressed by quantitative polymerase chain reaction (qPCR), suggesting that core 3-derived O-glycans do not significantly impact on commensal numbers. However, loss of core 3-derived O-glycans leads to exacerbated inflammation after DSS treatment, characterized by increased recruitment of inflammatory cells including macrophages and T cells (An et al. 2007). This was associated with more severe colonic mucosal destruction and increased overall morbidity and mortality (An et al. 2007).
Interestingly, in humans, a significant down-regulation of C3GnT has been reported in colon cancer patients (Iwai et al. 2005). We also saw increased baseline proliferation of epithelium in the absence of C3GnT (An et al. 2007). These observations suggested a role for loss of core 3-derived O-glycans in human cancer. Consistent with human studies, we found that core 3 O-glycan deficiency leads to increased size of colon tumors as well as greater levels of invasive carcinoma following azoxymethane-DSS treatment (An et al. 2007). These studies link core 3-derived mucin-type O-glycans as key components that fortify the mucus barrier to prevent bacterial-induced inflammation and inflammation-associated cancer following intestinal injury.
It was recently demonstrated that despite the similar expression pattern of C3GnT in human and mouse, murine Muc2 is primarily made up of core 1-derived O-glycans with core 3-derived glycans making up an estimated 1% of the total Muc2 O-glycome in mice (Thomsson et al. 2012). The relevance of these differences to human mucin barrier function and host–microbe homeostasis is currently unclear (Fu et al. 2011). Loss of core 3-derived O-glycans in mice likely only minimally reduces total Muc2 O-glycosylation levels and probably explains why spontaneous disease does not develop in the absence of C3GnT alone. Still, Thomsson et al. (2012) have observed that core 3-derived O-glycan structures have unique properties compared with core 1-derived O-glycans, including elongated glycan chains and reduced sulfation levels. Thus, in mice, it is likely that core 3-derived structures have subtle but important effects on host–microbe mutualism at baseline as evidenced by increased bacterial translocation and altered Muc2 homeostasis in the absence of C3GnT (An et al. 2007). Interestingly, there is evidence that core 3-derived O-glycans were overrepresented in the intestinal tract of IEC C1galt1−/− mice compared with WT mice (Fu et al. 2011). This is supported by recent in vitro studies where siRNA-mediated suppression of C1GalT1 led to increased expression of core 3-derived O-glycans (as well as sialyl Tn antigen) in human colon cancer cell lines (Barrow et al. 2013). These results suggest that C3GnT activity increases in the absence of C1galt1, either to compensate for total O-glycan loss or as a consequence of reduced competition of C3GnT with C1GalT1 for the Tn antigen (Barrow et al. 2013). In IEC C1galt1−/− mice, these core 3-derived O-glycans are also abnormally highly sialylated on Gal and GlcNAc residues (Thomsson et al. 2012). However, the role of these remaining core 3 structures in modifying the spontaneous disease in C1GalT1-deficient mice is still unknown. Our preliminary data using mice deficient in both core 1- and 3-derived O-glycans indicates a worsened disease compared with intestinal C1galt1 deficiency alone (K.S.B.B. and L.X., unpublished), suggesting a protective effect of core 3-derived O-glycans even when the major murine Muc2 O-glycans are lost.
Evidence for a protective role of mucin sulfation in colitis
Sulfation is a common mucin-type O-glycan modification. The enzymes responsible for glycan sulfation belong to the Gal/GalNAc/GlcNAc 6-O-sulfotransferase (GST) family, which are 6-O-sulfotransferases that transfer HSO4− from the universal sulfate donor 3′-phosphoadenosine 5′-phosphosulfate (PAPS) to C6 of Gal, GalNAc and GlcNAc, depending on the GST member (Brockhausen 2007; Brockhausen et al. 2009). In the large intestine, it was demonstrated that GlcNAc6ST-2 was the major GST family member responsible for sulfation of colonic Muc2 as there was a dramatic reduction in the histochemical staining of sulfomucins in colonic goblet cells in GlcNAc6ST-2 knockout mice (Tobisawa et al. 2010). liquid chromatography electrospray ionization tandem mass spectrometry (LC/ESI-MS/MS). analysis revealed that sulfation occurred primarily on core 2 structures (Tobisawa et al. 2010). Interestingly, the expression of GlcNAc6ST-2 was mediated by the short-chain fatty acid butyrate (Tobisawa et al. 2010), a metabolite derived from the digestion of fatty acids and glycans by the microbiota. However, this was only seen in the presence of epidermal growth factor, suggesting the cooperation of host- and microbial-derived factors in the regulation of O-glycan sulfation by this enzyme (Tobisawa et al. 2010). The physiological significance of mucin sulfation was addressed by DSS administration, where lack of GlcNAc6ST-2 led to increased infiltration of CD45+ inflammatory cells, including macrophages and neutrophils (Tobisawa et al. 2010). However, other disease parameters, including disease activity, histologic damage, bacterial translocation and mucus layer quality, unfortunately were not addressed (Tobisawa et al. 2010). Another study used mice deficient in the sulfate transporter NaS1, which is involved in the maintenance of circulating levels of SO4− (Dawson et al. 2009). NaS1−/− mice have reduced sulfation of goblet cell mucin in the colon (Dawson et al. 2009). The physiological function of this defect was addressed also with the DSS-colitis model, which revealed elevated disease activity in the absence of NaS1 compared with WT mice (Dawson et al. 2009). While these studies clearly link sulfation with protection against gastrointestinal insult, the mechanisms by which sulfated mucin-type O-glycans specifically mediate their protection are still unclear. Interestingly, higher sulfomucin levels in human colonic tissues correlated with greater representation of specific genera of sulfate-reducing bacteria including Desulfobacter, Desulfobulbus and Desulfotomaculum, relative to the predominant Desulfovibrio genus (Croix et al. 2011). Thus, examination of how sulfation of mucin-type O-glycans, especially on Muc2, impacts on the microbial community, mucus quality and colonic disease susceptibility presents an important area of further research.
The role of mucin-type O-glycans in shaping the intestinal ecosystem
While the above studies clearly establish mucin-type O-glycans as key contributors to the intestinal mucus barrier and protection from insult, it is clear that many outstanding questions remain in regard to how O-glycans specifically interact with bacteria and promote protective responses. O-glycans have long been appreciated as important components of the intestinal ecosystem (Hooper and Gordon 2001; Sonnenburg et al. 2004), but their precise influence on community structure and function is only beginning to be understood. To elucidate the specific influence of O-glycans on intestinal ecosystems is a daunting task due to the rapidly changing gut environment that results from diet and its effects on the microbiota (Turnbaugh et al. 2009; Wu et al. 2011). However, studies of O-glycan-deficient mice suggest that mucin-type O-glycans are highly pleiotropic in their role in regulating interactions with the microbiota. As described below, evidence points to mucin-type O-glycosylation as having several distinct, but not mutually exclusive, roles in promoting homeostasis with our resident microbes.
The role of mucin type-O-glycans in stabilizing the mucus barrier
The distal colon has the thickest mucus layer and also harbors the greatest density of bacteria. The mucus layer is breached in mice lacking intestinal core 1-derived O-glycans (Fu et al. 2011). This suggests that O-glycans impart structural stability to the mucus layer. This could occur by sterically blocking the MUC2 protein backbone from microbiota-derived proteases. The in vivo evidence for this has been only corollary; for example, the degree of loss of mucin-type O-glycans in mice seems to correlate with mucus barrier integrity and colitis susceptibility (core 1 > core 3 = core2/4 > WT). In vitro studies have suggested that glycosylated mucus is resistant to many proteases (Subramani et al. 2010), and recently it was shown that ppGalNAc-T3-mediated O-glycosylation of a human MUC2 peptide was important to protect the peptide from proteolytic attack by a cysteine protease derived from Porphyromonas gingivalis (van der Post et al. 2013). Alternatively, specific modifications of mucin-type O-glycans, including sulfation and sialation, are thought to directly inhibit bacterial glycosidase activity (Nieuw Amerongen et al. 1998). Both of these possibilities could explain the impaired mucus barrier in the absence of core 1 O-glycans, which are lacking in sulfated glycans normally found on core 2-branched structures (Tobisawa et al. 2010). Furthermore, the intestinal microbiota collectively exhibit a plethora of adhesins and glycosidase activities (fucosidase, sialidase, sulfatase and GalNAcase) that can efficiently break down mucin-type O-glycans into their monosaccharide components (Hoskins et al. 1985; Corfield et al. 1992). These structures can serve as decoys for bacterial adhesins that would otherwise bind to similar O-glycan structures of membrane-associated mucins. Therefore, a related function of O-glycans may be to function as decoy receptors to promote compartmentalization of bacteria into the lumen and prevent cell-associated glycan binding and degradation.
Mucin-type O-glycans and their regulation of microbial community composition
While O-glycans most likely impart structural stability to the mucus layers of the distal colon, they are increasingly appreciated as important in shaping the composition of the microbial communities within the gut. This was recently demonstrated in mice lacking the β1,4-N-acetylgalactoseaminyltransferase 2 (B4galnt2), which catalyzes the addition of GalNAc to an α-2,3-linked sialic acid-substituted Gal residue via a β1 → 4 linkage to form the Sd[a]/Cad antigen (Staubach et al. 2012). B4galnt2-deficient mice have an altered bacterial community structure in their intestines, as determined by 16S rRNA gene pyrosequencing (Staubach et al. 2012). While the Sd[a]/Cad antigen is found mainly in human colonic MUC2, most likely on core 3-derived O-glycans (Robbe et al. 2004), lack of B4galnt2 in mice had its greatest effect on community composition in the ileum (Staubach et al. 2012). Interestingly, Helicobacter sp. were dramatically underrepresented in the B4galnt2-null mice, suggesting that Sd[a]/Cad epitopes are important determinants of pathogen colonization (Staubach et al. 2012).
Human secretor status has also recently been linked to microbial community composition in the gut. Secretors represent individuals with a functional FUT2 gene, which encodes galactoside 2-alpha-l-fucosyltransferase 2 (FUT-2). FUT2 catalyzes the transfer of l-fucose via an α1,2 linkage to Gal residues on histo-blood group antigen precursors (e.g., type1 Galβ1-3GlcNAcβ1-R) on mucin-type O-glycans leading to expression of H-antigens in mucosal and salivary secretions including mucins (Ikehara et al. 2001). These H-antigens can be further substituted by blood group A and B transferases to form the blood group A and B antigens and by Lewis transferase (α1-3,4 fucosyltransferase, FUT3) to produce Lewis determinants (e.g., Leb or Ley). About 20% of Caucasians have homozygous null mutation (W143X) in FUT2 and therefore cannot synthesize ABH or Le (b or y) antigens in mucin-type O-glycans and are thus termed “non-secretors” (Ikehara et al. 2001). Nonsecretors possess a significantly distinct microbiota community composition compared with secretors (Rausch et al. 2011). Among the most notable distinctions are an increased association of nonsecretors with the genus Prevotella (Rausch et al. 2011). This has implications for mucosal homeostasis, as some Prevotella sp. express a unique mucin-desulfating glycosidase that can hydrolyze terminal 6-SO3-GlcNAc residues on mucin-type O-glycans (Rho et al. 2005), which could compromise mucus barrier integrity. There were also decreased associations of known health-promoting bacteria, such as members of the genera Lactobacillus (Rausch et al. 2011) and Bifidobacterium (Wacklin et al. 2011) compared with secretors. Interestingly, FUT2 mutations have recently been associated with IBD (McGovern et al. 2010). Since altered composition of the microbiota is thought to contribute to the pathogenesis of IBD (Manichanh et al. 2012), these studies suggest that alterations in mucin-type O-glycans potentially link dysbiosis of the microbiota with the development of IBD. Consistent with this, members of the Lachnospiraceae family, which are also strongly associated with nonsecretor status (Rausch et al. 2011), have been shown to promote inflammation in Crohn's Disease (CD) (Duck et al. 2007). Despite these findings, Fut2-deficient mice, a murine model of nonsecretor status, have not been reported to display spontaneous IBD to date (Iwamori and Domino 2004; Hurd et al. 2005; Magalhaes et al. 2009), although this may reflect the need for an additional genetic or environmental trigger. Lastly, humans expressing blood group B antigens exhibited greater diversity of Eubacterium and Clostridium species compared with individuals expressing nonblood group B antigens (Makivuokko et al. 2012). These results demonstrate that subtle distinctions in the structure of O-glycans can influence microbial species composition, which may impact on susceptibility to intestinal diseases.
The basis of O-glycan-mediated microbial selection is still unclear but probably involves binding of bacterial lectin-like adhesins to specific structures on mucin-type O-glycans. For example, Lactobacillus has been shown to bind specifically to blood group A and B epitopes on human colonic mucin via its surface-bound GAPDH (Kinoshita et al. 2008), which may explain its association with healthy secretors (Rausch et al. 2011). As described below for Escherichia coli, the O-glycans present in secreted mucins may also impact on the ability of some bacteria to thrive after they colonize (Chang et al. 2004), suggesting a nutritional basis for selection. It is also possible that mucins may have direct antimicrobial activity. While this has only been demonstrated in the gastric mucosa so far with the discovery of an α1,4 GlcNAc capped core-2-derived O-glycan on MUC6 that can directly kill Helicobacter pylori (Kawakubo et al. 2004; Kobayashi et al. 2009), it is plausible that similar antimicrobial O-glycans can exist in the intestine.
The influence of mucin-type O-glycans on metabolic function of the microbiota
The commensal microbiota provides many benefits to health including protection from pathogens (Kamada et al. 2012) and energy from the diet (O'Hara and Shanahan 2006). Mounting evidence is revealing mucin-type O-glycans as important contributors to this mutualism by impacting on the biology of microorganisms directly, which can influence which bacteria thrive and how they ultimately interact with the host tissues.
Bacteroides species are anaerobic gram-negative bacteria and dominant members of the mammalian microbiota (Ley et al. 2008). Foundational studies from the lab of Jeffrey Gordon using the prominent gut symbiont Bacteroides thetaiotaomicron have provided key insights into the role of mucosal O-glycans in function of the microbiota. B. thetaiotaomicron harbors 88 different polysaccharide utilization loci (PUL) in its genome to degrade complex carbohydrates from diet-derived (e.g., plants, milk) or host-derived (e.g., mucin) glycans (Martens et al. 2008). Mucin-type O-glycans can directly induce 16 of these PUL (Martens et al. 2009b). Interestingly, the latter PUL encode genes that endow B. thetaiotaomicron with the ability to forage mucosal O-glycans for nutrients when glycans from dietary (e.g., plant) polysaccharides are low in abundance (Sonnenburg 2005). Moreover, mucin-type O-glycans are important for fitness and transmission of these bacteria from mother to offspring (Martens et al. 2008). Furthermore, B. thetaiotaomicron surveys the gut mucosal glycan landscape for l-fucose availability. If fucose abundance is low, B. thetaiotaomicron uses its FucR sensor to increase production of a soluble secreted factor that can induce the expression of Fut2 in IEC (Hooper et al. 1999; Meng et al. 2007). This leads to expression of α1,2 fucosylated glycans by IEC, which is then harvested by B. thetaiotaomicron for metabolic use (Hooper et al. 1999). Thus, mucin-type O-glycans are a critical resource utilized by this commensal to enable it to thrive when diet-derived glycans are compromised as well as to persist in mammalian populations. In turn, B. thetaiotaomicron can affect host responses in beneficial ways, such as stimulating angiogenesis to increase absorptive capacity of the intestine (Stappenbeck et al. 2002), providing energy in the form of short-chain fatty acids (e.g., butyrate, propionate and acetate) elaborated as end-products of glycan fermentation (Comstock 2009) and promoting mucosal homeostasis by both limiting inflammatory tone of the epithelium (Kelly et al. 2004) and stimulating production of epithelial antimicrobials to potentially reduce overall bacterial load at mucosal surfaces (Hooper et al. 2003).
Induction of binding and degradation of mucin-type O-glycans by B. thetaiotaomicron also helps to regulate genetic programs that assist in outer capsule synthesis (Martens et al. 2009a). While the biologic function of this capsule synthesis in B. thetaiotaomicron is not clear, insights can be gained from a related symbiont Bacteroides fragilis. The Comstock laboratory has shown that B. fragilis, like B. thetaiotaomicron, has genetic machinery to degrade and utilize glycans, including mucin-type O-glycans, for capsular polysaccharide synthesis (Coyne et al. 2005; Comstock 2009) which are collectively required for optimal colonization and maintenance in the gut (Coyne et al. 2008; Liu et al. 2008). Additional work by the Mazmanian and Kasper laboratories have shown numerous effects of one specific capsular polysaccharide, Polysaccharide A (PSA), on B. fragilis colonization, host immune development and protection from disease. Specifically, PSA can be sensed by TLR2 on CD4+ T cells, which can induce them to differentiate into immunosuppressive regulatory T-cells (Tregs) that secrete interleukin-10 (Round and Mazmanian 2010). This in turn is critical for B. fragilis to colonize a unique niche in the colon and persist (Round et al. 2011). Once colonized, B. fragilis can release PSA-bound outer membrane vesicles that are sensed by TLR2 on dendritic cells, which subsequently induce the differentiation of these anti-inflammatory Tregs in the intestine (Shen et al. 2012). These effects of PSA have been shown to have a profound role in ameliorating colitis severity in response to potentially colitogenic bacteria and chemical agents (Mazmanian et al. 2008; Round and Mazmanian 2010; Shen et al. 2012). Although the relative contribution of dietary vs. host-derived (e.g., mucin) sources of glycans for utilization by B. fragilis is unclear, B. fragilis has recently been shown to colonize murine mucus in vivo and directly bind to purified soluble mucus (Huang et al. 2011). While the authors of this study conclude that most B. fragilis was found in the lumen with smaller numbers in the mucus layer (Huang et al. 2011), an alternative interpretation is that most B. fragilis was localized to the outer mucus layer which is abundant in soluble mucins, with few, remarkably, colonizing the normally impenetrable inner mucus layer. Importantly, the ability of B. fragilis to degrade porcine colonic mucin O-glycans in vitro was limited but was enough to support its growth in media when purified colonic mucin was used as the sole carbon source (Roberton and Stanley 1982). Thus, mucin-type O-glycans contribute both directly or indirectly to robust colonization of the gut by B. fragilis and production of commensal-derived symbiotic molecules such as PSA.
Members of the Bifidobacteria genus, which are gram-positive obligate anaerobes, have numerous health-promoting interactions with the host. Among these include regulating pathogen colonization (Fanning et al. 2012) and possibly reducing factors associated with colitis-associated cancer (Femia et al. 2002). Mucin-type O-glycans play a key role in metabolism of this species and its establishment in the intestinal tract. For example, Bifidobacterium bifidum resides in the infant gut and has been shown to harbor a rich collection of glycoside hydrolases in its genome that allow it to degrade and metabolize glycans from human mucins (Turroni et al. 2010, 2011). One of these enzymes is endo-α-N-acetylgalatosaminidase, a novel glycoside hydrolase family member that can hydrolyze O-glycosidic linkages between GalNAc and Ser/Thr residues of core 1 structures (Fujita et al. 2005). In addition, B. bifidum possesses another unique glycoside hydrolase family member called afcA, which is a 1,2 α-l-fucosidase that releases terminal fucose from glycans by hydrolyzing α-(1-2) fucosidic linkages (Katayama et al. 2004). Bifidobacterium sp. can grow in mucins, and the induction of these glycoside hydrolase genes can be induced by mucin binding (Ruas-Madiedo et al. 2008). Bifidobacterium bifidum also carries genes that can potentially degrade core 2, 3 and 4 structures (Turroni et al. 2011). These studies collectively reveal the intimate relationship between mucin-type O-glycans and Bifidobacterium biology, with significant implications for overall health of the host.
Escherichia coli is the dominant facultative anaerobe of the mammalian intestinal tract (Chang et al. 2004). When exposed to murine cecal mucus, E. coli upregulates a spectrum of genes involved in phospholipid and amino acid biosynthesis as well as carbohydrate metabolism (Chang et al. 2004). Interestingly, in a systematic analysis of the role of each of these genes in host colonization, it was shown that only defects in the carbohydrate utilization loci affected the ability of E. coli to colonize or persist in the murine intestine (Chang et al. 2004). Importantly, E. coli displays a preference for specific O-glycan-derived monosaccharides at different stages of colonization. Genes involved in catabolism of GlcNAc were upregulated during the initiation (log) phase of colonization, and genes involved in fucose and sialic acid catabolism were required for the maintenance (stationary) phases (Chang et al. 2004). These results suggest that commensal E. coli depends on mucin-type O-glycans to fine-tune responses that assist in its colonization and establishment of the intestine. Moreover, utilization of these glycans may provide a nutritional basis for how these commensal species can outcompete pathogens (Leatham et al. 2009; Maltby et al. 2013).
Finally, the interaction of mucin-type O-glycans with some commensal species can influence the growth of other commensal species. In a recent study, Akkermansia muciniphila, a prominent mucin degrader found in the healthy colon (Derrien et al. 2008; Arumugam et al. 2011), was able to degrade MUC2 O-glycans in vitro, whereas Bacteroides vulgatus was not (Png 2010). When these two bacteria were cultured together in the presence of mature MUC2 as the sole carbon source, growth of B. vulgatus was enhanced almost four-fold (Png 2010). Similar results were seen when B. vulgatus was co-cultured with two other mucolytic bacterial species, Ruminococcus torques and Ruminococcus gnavus (Png 2010). Interestingly, A muciniphila was able to stimulate the growth of all species but itself did not benefit from mucus degradation, sometimes showing reduced growth, suggesting that mucosal O-glycans can mediate altruistic-like behavior of other bacterial species under specific conditions.
Mucin-type O-glycans as scaffolds to position host-derived nonmucin factors in the lumen
While small intestinal mucus lacks an inner layer that separates commensal bacteria from the epithelium, the Hooper laboratory has demonstrated that commensal bacteria can induce the expression and secretion of RegIIIγ, an antimicrobial C-type lectin (Cash et al. 2006), which can promote a bacteria-free zone adjacent to the epithelial surface (Vaishnava et al. 2011). This suggests a mechanism different from an impenetrable mucus layer to segregate luminal bacteria in the small intestine. Interestingly, isolated small intestinal mucus contained an abundant array of antimicrobial molecules derived from Paneth cells, which were adept at directly killing pathogenic and commensal bacteria (Meyer-Hoffert et al. 2008), indicating that mucus can function as molecular scaffold for these antimicrobials. It is likely that mucin-type O-glycans are important in this carrier process; however, the degree to which this is true is not known. Similarly, secretory Immunoglobulin-A (SIgA) is important in regulating bacterial interactions in the intestinal tract (reviewed in Macpherson et al. 2012). This is thought in part to be due to SIgA being localized to the mucus layer via secretory component to mediate its protective functions (Phalipon et al. 2002). Therefore, mucin-type O-glycans are likely important for effective SIgA function. Proteomic analysis of the colonic mucus layers has similarly shown abundant proteins residing in the inner and outer mucus layers (Johansson et al. 2009). Many of these were postulated to be released from cells sloughed-off from the intestinal tissue during physiologic turnover, but over 40 were predicted to be secreted products, mainly host-derived residing in the mucus layer, including plasma and mucin-binding proteins (Johansson et al. 2009). Currently, the physiologic significance of these proteins or their positioning in the mucus is not known nor what maintains their localization in the mucus. However, it is likely that O-glycans are important in this overall process as they contribute to the gel-like trapping and rheological properties of mucus (Thornton et al. 2008), and relatively few molecules have been demonstrated to covalently bind, or even have access to, the protein core of secreted Muc2 (Johansson et al. 2009). Moreover, the strong negative charge due to substitution of terminal epitopes with sialic acid or sulfate residues could lead to noncovalent interactions with positively charged secreted proteins. The role of Muc2 O-glycosylation in assisting in function of nonmucin components of secreted mucus could be directly tested in vivo using O-glycan-deficient mice to assess the effect of Muc2 O-glycan loss on localization of target molecules such as IgA, RegIIIγ and other antimicrobial peptides in the lumen.
Conclusions and perspectives
Mucin-type O-glycans have long been considered to have critical roles in influencing host–microbe interactions at mucosal sites, especially the intestinal tract. However, only in the last decade did we begin to understand the nature and extent to which mucin-type O-glycans impact on homeostasis in the gut and their role in protection from intestinal diseases such as IBD. While the studies outlined in this review represent major steps forward in our current knowledge of the biological functions of mucin-type O-glycans, much of our mechanistic understanding of how O-glycans promote mucus barrier function and shaping microbial community composition is still speculative at best. Conversely, while much knowledge has been gathered with respect to how O-glycans are utilized by specific members of our gut (e.g., Bacteroides spp.), limited work has been done on most bacterial species that have been studied, so the field is wide open to understand how O-glycans impact on these species (e.g., A. muciniphila, Prevotella spp.). Although beyond the scope of this review, an additional layer of complexity is the modulation of glycosylation that can occur in various diseases including IBD (Larsson et al. 2011) or intestinal infections (reviewed in McGuckin et al. 2011), which can likely have secondary effects on mucin function and host–microbe interactions. Ultimately, modulation of O-glycosylation status during chronic inflammatory diseases or by somatic or inherited mutation in genes impacting on O-glycosylation will likely have a profound impact on host–microbe mutualism in the gut. Therefore, the current challenge put before mucosal glycobiologists is to identify precisely how O-glycans influence interactions with the microbiota under physiologic and pathologic conditions. Only then can we interpret the meaning of specific glycosylation events associated with these conditions and intervene as necessary.
Funding
This work was supported by NIH grants RR018758 and R01DK085691 (to L. Xia); and by a Research Fellows Award 285148 (to K. Bergstrom) from the Crohn's and Colitis Foundation of America.
Conflict of interest
None declared.
Abbreviations
DSS, dextran sodium sulfate; FUT-2, fucosyltransferase 2; Gal, galactose; GlcNAc, N-acetylglucosamine; IBD, inflammatory bowel disease; IEC, intestinal epithelial cells; MUC2, Mucin-2; O-GalNAc, O-linked N-acetylgalactosamine; ppGalNAcTs, polypeptidyl GalNAc transferases; PUL, polysaccharide utilization loci; SIgA, secretory Immunoglobulin-A; TLR, Toll-like receptor; TM, tamoxifen; UC, ulcerative colitis; UDP, uridine diphosphate.
References
- Allen A, Hutton DA, Pearson JP. The MUC2 gene product: A human intestinal mucin. Int J Biochem Cell B. 1998;30:797–801. doi: 10.1016/s1357-2725(98)00028-4. [DOI] [PubMed] [Google Scholar]
- An G, Wei B, Xia B, McDaniel JM, Ju T, Cummings RD, Braun J, Xia L. Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J Exp Med. 2007;204:1417–1429. doi: 10.1084/jem.20061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende D, Fernandes G, Tap J, Bruls T, Batto J, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998;273:18857–18863. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: Thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 2001;11:633–644. doi: 10.1093/glycob/11.8.633. [DOI] [PubMed] [Google Scholar]
- Backstrom M, Ambort D, Thomsson E, Johansson ME, Hansson GC. Increased understanding of the biochemistry and biosynthesis of MUC2 and other gel-forming mucins through the recombinant expression of their protein domains. Mol Biotechnol. 2013;54(2):250–256. doi: 10.1007/s12033-012-9562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafna S, Kaur S, Batra SK. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893–2904. doi: 10.1038/onc.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow H, Tam B, Duckworth CA, Rhodes JM, Yu LG. Suppression of core 1 Gal-transferase is associated with reduction of TF and reciprocal increase of Tn, sialyl-Tn and core 3 glycans in human colon cancer cells. PLoS One. 2013;8:e59792. doi: 10.1371/journal.pone.0059792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhuizen MF, Fukuda M. Expression cloning of a cDNA encoding UDP-GlcNAc:Gal beta 1–3-GalNAc-R (GlcNAc to GalNAc) beta 1-6GlcNAc transferase by gene transfer into CHO cells expressing polyoma large tumor antigen. Proc Natl Acad Sci USA. 1992;89:9326–9330. doi: 10.1073/pnas.89.19.9326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockhausen I. Biosynthesis of mucin-type O-glycans. In: Johannis PK, editor. Comprehensive Glycoscience. Oxford: Elsevier; 2007. pp. 33–59. [Google Scholar]
- Brockhausen I, Schachter H, Stanley P. O-GalNAc glycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2nd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009. pp. 115–128. Chapter 9. [Google Scholar]
- Byrd J, Bresalier R. Mucins and mucin binding proteins in colorectal cancer. Cancer Metast Rev. 2004;23:77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- Cash HL, Whitham CV, Behrendt CL, Hooper LV. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science. 2006;313:1126–1130. doi: 10.1126/science.1127119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang DE, Smalley DJ, Tucker DL, Leatham MP, Norris WE, Stevenson SJ, Anderson AB, Grissom JE, Laux DC, Cohen PS, et al. Carbon nutrition of Escherichia coli in the mouse intestine. Proc Natl Acad Sci USA. 2004;101:7427–7432. doi: 10.1073/pnas.0307888101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock LE. Importance of glycans to the host-bacteroides mutualism in the mammalian intestine. Cell Host Microbe. 2009;5:522–526. doi: 10.1016/j.chom.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Corfield AP, Wagner SA, Clamp JR, Kriaris MS, Hoskins LC. Mucin degradation in the human colon: Production of sialidase, sialate O-acetylesterase, N-acetylneuraminate lyase, arylesterase, and glycosulfatase activities by strains of fecal bacteria. Infect Immun. 1992;60:3971–3978. doi: 10.1128/iai.60.10.3971-3978.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Chatzidaki-Livanis M, Paoletti LC, Comstock LE. Role of glycan synthesis in colonization of the mammalian gut by the bacterial symbiont Bacteroides fragilis. Proc Natl Acad Sci USA. 2008;105:13099–13104. doi: 10.1073/pnas.0804220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne MJ, Reinap B, Lee MM, Comstock LE. Human symbionts use a host-like pathway for surface fucosylation. Science. 2005;307:1778–1781. doi: 10.1126/science.1106469. [DOI] [PubMed] [Google Scholar]
- Croix JA, Carbonero F, Nava GM, Russell M, Greenberg E, Gaskins HR. On the relationship between sialomucin and sulfomucin expression and hydrogenotrophic microbes in the human colonic mucosa. PLoS One. 2011;6:e24447. doi: 10.1371/journal.pone.0024447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Huxley S, Gardiner B, Tran T, McAuley JL, Grimmond S, McGuckin MA, Markovich D. Reduced mucin sulfonation and impaired intestinal barrier function in the hyposulfataemic NaS1 null mouse. Gut. 2009;58:910–919. doi: 10.1136/gut.2007.147595. [DOI] [PubMed] [Google Scholar]
- Derrien M, Collado MC, Ben-Amor K, Salminen S, de Vos WM. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl Environ Microbiol. 2008;74:1646–1648. doi: 10.1128/AEM.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duck LW, Walter MR, Novak J, Kelly D, Tomasi M, Cong Y, Elson CO. Isolation of flagellated bacteria implicated in Crohn's disease. Inflamm Bowel Dis. 2007;13:1191–1201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- Fanning S, Hall LJ, Cronin M, Zomer A, MacSharry J, Goulding D, O'Connell Motherway M, Shanahan F, Nally K, Dougan G, et al. Bifidobacterial surface-exopolysaccharide facilitates commensal-host interaction through immune modulation and pathogen protection. Proc Natl Acad Sci USA. 2012;109:2108–2113. doi: 10.1073/pnas.1115621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femia AP, Luceri C, Dolara P, Giannini A, Biggeri A, Salvadori M, Clune Y, Collins KJ, Paglierani M, Caderni G. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis. 2002;23:1953–1960. doi: 10.1093/carcin/23.11.1953. [DOI] [PubMed] [Google Scholar]
- Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Oura F, Nagamine N, Katayama T, Hiratake J, Sakata K, Kumagai H, Yamamoto K. Identification and molecular cloning of a novel glycoside hydrolase family of core 1 type O-glycan-specific endo-α-N-acetylgalactosaminidase from Bifidobacterium longum. J Biol Chem. 2005;280:37415–37422. doi: 10.1074/jbc.M506874200. [DOI] [PubMed] [Google Scholar]
- Godl K, Johansson ME, Lidell ME, Morgelin M, Karlsson H, Olson FJ, Gum JR, Jr, Kim YS, Hansson GC. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Gordon JI. Glycans as legislators of host-microbial interactions: Spanning the spectrum from symbiosis to pathogenicity. Glycobiology. 2001;11:1R–10R. doi: 10.1093/glycob/11.2.1r. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4:269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Xu J, Falk PG, Midtvedt T, Gordon JI. A molecular sensor that allows a gut commensal to control its nutrient foundation in a competitive ecosystem. Proc Natl Acad Sci USA. 1999;96:9833–9838. doi: 10.1073/pnas.96.17.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskins L, Agustines M, McKee W, Boulding E, Kriaris M, Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985;75:944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JY, Lee SM, Mazmanian SK. The human commensal Bacteroides fragilis binds intestinal mucin. Anaerobe. 2011;17:137–141. doi: 10.1016/j.anaerobe.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd EA, Holmen JM, Hansson GC, Domino SE. Gastrointestinal mucins of Fut2-null mice lack terminal fucosylation without affecting colonization by Candida albicans. Glycobiology. 2005;15:1002–1007. doi: 10.1093/glycob/cwi089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara Y, Nishihara S, Yasutomi H, Kitamura T, Matsuo K, Shimizu N, Inada K-I, Kodera Y, Yamamura Y, Narimatsu H, et al. Polymorphisms of two fucosyltransferase genes (Lewis and Secretor genes) involving type I Lewis antigens are associated with the presence of anti-Helicobacter pylori IgG antibody. Cancer Epidem Biomar. 2001;10:971–977. [PubMed] [Google Scholar]
- Iwai T, Inaba N, Naundorf A, Zhang Y, Gotoh M, Iwasaki H, Kudo T, Togayachi A, Ishizuka Y, Nakanishi H, et al. Molecular cloning and characterization of a novel UDP-GlcNAc:GalNAc-peptide beta1,3-N-acetylglucosaminyltransferase (beta 3Gn-T6), an enzyme synthesizing the core 3 structure of O-glycans. J Biol Chem. 2002;277:12802–12809. doi: 10.1074/jbc.M112457200. [DOI] [PubMed] [Google Scholar]
- Iwai T, Kudo T, Kawamoto R, Kubota T, Togayachi A, Hiruma T, Okada T, Kawamoto T, Morozumi K, Narimatsu H. Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc Natl Acad Sci USA. 2005;102:4572–4577. doi: 10.1073/pnas.0407983102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamori M, Domino SE. Tissue-specific loss of fucosylated glycolipids in mice with targeted deletion of alpha(1,2)fucosyltransferase genes. Biochem J. 2004;380:75–81. doi: 10.1042/BJ20031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH, et al. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3635–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjovall H, et al. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2012-303207. doi:10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Gustafsson JK, Sjoberg KE, Petersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Holmen Larsson JM, Hansson GC. Microbes and Health Sackler Colloquium: The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson ME, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013 doi: 10.1038/nrgastro.2013.35. doi:10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MEV, Thomsson KA, Hansson GC. Proteomic analyses of the two mucus layers of the colon barrier reveal that their main component, the Muc2 mucin, is strongly bound to the Fcgbp protein. J Proteome Res. 2009;8:3549–3557. doi: 10.1021/pr9002504. [DOI] [PubMed] [Google Scholar]
- Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 beta 3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju T, Cummings RD, Canfield WM. Purification, characterization, and subunit structure of rat core 1 Beta1,3-galactosyltransferase. J Biol Chem. 2002;277:169–177. doi: 10.1074/jbc.M109056200. [DOI] [PubMed] [Google Scholar]
- Kamada N, Kim Y-G, Sham HP, Vallance BA, Puente JL, Martens EC, Nunez G. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science. 2012;336:1325–1329. doi: 10.1126/science.1222195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Sakuma A, Kimura T, Makimura Y, Hiratake J, Sakata K, Yamanoi T, Kumagai H, Yamamoto K. Molecular cloning and characterization of Bifidobacterium bifidum 1,2-α-fucosidase (AfcA), a novel inverting glycosidase (glycoside hydrolase family 95) J Bacteriol. 2004;186:4885–4893. doi: 10.1128/JB.186.15.4885-4893.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003–1006. doi: 10.1126/science.1099250. [DOI] [PubMed] [Google Scholar]
- Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Wakahara N, Watanabe M, Kawasaki T, Matsuo H, Kawai Y, Kitazawa H, Ohnuma S, Miura K, Horii A, et al. Cell surface glyceraldehyde-3-phosphate dehydrogenase (GAPDH) of Lactobacillus plantarum LA 318 recognizes human A and B blood group antigens. Res Microbiol. 2008;159:685–691. doi: 10.1016/j.resmic.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Lee H, Nakayama J, Fukuda M. Carbohydrate-dependent defense mechanisms against Helicobacter pylori infection. Curr Drug Metab. 2009;10:29–40. doi: 10.2174/138920009787048428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson JM, Karlsson H, Crespo JG, Johansson ME, Eklund L, Sjovall H, Hansson GC. Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm Bowel Dis. 2011;17:2299–2307. doi: 10.1002/ibd.21625. [DOI] [PubMed] [Google Scholar]
- Leatham MP, Banerjee S, Autieri SM, Mercado-Lubo R, Conway T, Cohen PS. Precolonized human commensal Escherichia coli strains serve as a barrier to E. coli O157:H7 growth in the streptomycin-treated mouse intestine. Infect Immun. 2009;77:2876–2886. doi: 10.1128/IAI.00059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, Schlegel ML, Tucker TA, Schrenzel MD, Knight R, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19:315–337. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CH, Lee SM, Vanlare JM, Kasper DL, Mazmanian SK. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc Natl Acad Sci USA. 2008;105:3951–3956. doi: 10.1073/pnas.0709266105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Magalhaes A, Gomes J, Ismail MN, Haslam SM, Mendes N, Osorio H, David L, Le Pendu J, Haas R, Dell A, et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology. 2009;19:1525–1536. doi: 10.1093/glycob/cwp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnet AD, Fukuda M. Expression of the large I antigen forming beta-1,6-N-acetylglucosaminyltransferase in various tissues of adult mice. Glycobiology. 1997;7:285–295. doi: 10.1093/glycob/7.2.285. [DOI] [PubMed] [Google Scholar]
- Makivuokko H, Lahtinen S, Wacklin P, Tuovinen E, Tenkanen H, Nikkila J, Bjorklund M, Aranko K, Ouwehand A, Matto J. Association between the ABO blood group and the human intestinal microbiota composition. BMC Microbiol. 2012;12:94. doi: 10.1186/1471-2180-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltby R, Leatham-Jensen MP, Gibson T, Cohen PS, Conway T. Nutritional basis for colonization resistance by human commensal Escherichia coli strains HS and Nissle 1917 against E. coli O157:H7 in the mouse intestine. PLoS One. 2013;8:e53957. doi: 10.1371/journal.pone.0053957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:599–608. doi: 10.1038/nrgastro.2012.152. [DOI] [PubMed] [Google Scholar]
- Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe. 2008;4:447–457. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Roth R, Heuser JE, Gordon JI. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J Biol Chem. 2009a;284:18445–18457. doi: 10.1074/jbc.M109.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens EC, Roth R, Heuser JE, Gordon JI. Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J Biol Chem. 2009b;284:18445–18457. doi: 10.1074/jbc.M109.008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McGovern DPB, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, Ippoliti A, Vasiliauskas E, Berel D, Derkowski C, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet. 2010;19:3468–3476. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuckin MA, Lindën SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Micro. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- Meng D, Newburg DS, Young C, Baker A, Tonkonogy SL, Sartor RB, Walker WA, Nanthakumar NN. Bacterial symbionts induce a FUT2-dependent fucosylated niche on colonic epithelium via ERK and JNK signaling. Am J Physiol Gastrointest Liver Physiol. 2007;293:G780–G787. doi: 10.1152/ajpgi.00010.2007. [DOI] [PubMed] [Google Scholar]
- Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Putsep K, Andersson M. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut. 2008;57:764–771. doi: 10.1136/gut.2007.141481. [DOI] [PubMed] [Google Scholar]
- Nieuw Amerongen AV, Bolscher JG, Bloemena E, Veerman EC. Sulfomucins in the human body. Biol Chem. 1998;379:1–18. doi: 10.1515/bchm.1998.379.1.1. [DOI] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol. 2010;26:547–553. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- Phalipon A, Cardona A, Kraehenbuhl JP, Edelman L, Sansonetti PJ, Corthesy B. Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity. 2002;17:107–115. doi: 10.1016/s1074-7613(02)00341-2. [DOI] [PubMed] [Google Scholar]
- Png CW. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- Podolsky DK, Fournier DA. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988;95:379–387. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- Rausch P, Rehman A, Künzel S, Häsler R, Ott SJ, Schreiber S, Rosenstiel P, Franke A, Baines JF. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA. 2011;108:19030–19035. doi: 10.1073/pnas.1106408108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho JH, Wright DP, Christie DL, Clinch K, Furneaux RH, Roberton AM. A novel mechanism for desulfation of mucin: Identification and cloning of a mucin-desulfating glycosidase (sulfoglycosidase) from Prevotella strain RS2. J Bacteiol. 2005;187:1543–1551. doi: 10.1128/JB.187.5.1543-1551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe C, Capon C, Coddeville B, Michalski J-C. Structural diversity and specific distribution of O-glycans in normal human mucins along the intestinal tract. Biochem J. 2004;384:307–316. doi: 10.1042/BJ20040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton AM, Stanley RA. In vitro utilization of mucin by Bacteroides fragilis. Appl Environ Microbiol. 1982;43:325–330. doi: 10.1128/aem.43.2.325-330.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas-Madiedo P, Gueimonde M, Fernández-García Ma, de los Reyes-Gavilán CG, Margolles A. Mucin degradation by Bifidobacterium strains isolated from the human intestinal microbiota. Appl Environ Microbiol. 2008;74:1936–1940. doi: 10.1128/AEM.02509-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. doi: 10.1053/j.gastro.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Schwerbrock NM, Makkink MK, van der Sluis M, Buller HA, Einerhand AW, Sartor RB, Dekker J. Interleukin 10-deficient mice exhibit defective colonic Muc2 synthesis before and after induction of colitis by commensal bacteria. Inflamm Bowel Dis. 2004;10:811–823. doi: 10.1097/00054725-200411000-00016. [DOI] [PubMed] [Google Scholar]
- Schwientek T, Yeh JC, Levery SB, Keck B, Merkx G, van Kessel AG, Fukuda M, Clausen H. Control of O-glycan branch formation. Molecular cloning and characterization of a novel thymus-associated core 2 beta1, 6-n-acetylglucosaminyltransferase. J Biol Chem. 2000;275:11106–11113. doi: 10.1074/jbc.275.15.11106. [DOI] [PubMed] [Google Scholar]
- Shen Y, Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg JL. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–1959. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Angenent LT, Gordon JI. Getting a grip on things: How do communities of bacterial symbionts become established in our intestine? Nat Immunol. 2004;5:569–573. doi: 10.1038/ni1079. [DOI] [PubMed] [Google Scholar]
- Spiro RG. Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA. 2002;99:15451–15455. doi: 10.1073/pnas.202604299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staubach F, Kunzel S, Baines AC, Yee A, McGee BM, Backhed F, Baines JF, Johnsen JM. Expression of the blood-group-related glycosyltransferase B4galnt2 influences the intestinal microbiota in mice. ISME J. 2012;6:1345–1355. doi: 10.1038/ismej.2011.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Ismail MN, Lee SH, Luu Y, Ramirez K, Haslam SM, Ho SB, Dell A, Fukuda M, Marth JD. Glycosyltransferase function in core 2-type protein O glycosylation. Mol Cell Biol. 2009;29:3770–3782. doi: 10.1128/MCB.00204-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Lee SH, Ismail MN, Fukuda M. Characterization of mice with targeted deletion of the gene encoding core 2 beta1,6-N-acetylglucosaminyltransferase-2. Methods Enzymol. 2010;479:155–172. doi: 10.1016/S0076-6879(10)79009-1. [DOI] [PubMed] [Google Scholar]
- Subramani DB, Johansson ME, Dahlen G, Hansson GC. Lactobacillus and Bifidobacterium species do not secrete protease that cleaves the MUC2 mucin which organises the colon mucus. Benef Microbes. 2010;1:343–350. doi: 10.3920/BM2010.0039. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsson KA, Holmen-Larsson JM, Angstrom J, Johansson ME, Xia L, Hansson GC. Detailed O-glycomics of the Muc2 mucin from colon of wild-type, core 1- and core 3-transferase-deficient mice highlights differences compared with human MUC2. Glycobiology. 2012;22:1128–1139. doi: 10.1093/glycob/cws083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–486. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- Tobisawa Y, Imai Y, Fukuda M, Kawashima H. Sulfation of colonic mucins by N-acetylglucosamine 6-O-sulfotransferase-2 and its protective function in experimental colitis in mice. J Biol Chem. 2010;285:6750–6760. doi: 10.1074/jbc.M109.067082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: A metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1 doi: 10.1126/scitranslmed.3000322. 6ra14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F, Bottacini F, Foroni E, Mulder I, Kim J-H, Zomer A, Sánchez B, Bidossi A, Ferrarini A, Giubellini V, et al. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc Natl Acad Sci USA. 2010;107:19514–19519. doi: 10.1073/pnas.1011100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turroni F, Milani C, van Sinderen D, Ventura M. Genetic strategies for mucin metabolism in Bifidobacterium bifidum PRL2010: An example of possible human-microbe co-evolution. Gut Microbes. 2011;2:183–189. doi: 10.4161/gmic.2.3.16105. [DOI] [PubMed] [Google Scholar]
- Tytgat KM, van der Wal JW, Einerhand AW, Buller HA, Dekker J. Quantitative analysis of MUC2 synthesis in ulcerative colitis. Biochem Biophys Res Commun. 1996;224:397–405. doi: 10.1006/bbrc.1996.1039. [DOI] [PubMed] [Google Scholar]
- Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, Ley R, Wakeland EK, Hooper LV. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Post S, Subramani DB, Backstrom M, Johansson ME, Vester-Christensen MB, Mandel U, Bennett EP, Clausen H, Dahlen G, Sroka A, et al. Site-specific O-glycosylation on the MUC2 mucin inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB) J Biol Chem. 2013 doi: 10.1074/jbc.M113.459479. 10.1074/jbc.M113.459479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- van Klinken BJ, Einerhand AW, Duits LA, Makkink MK, Tytgat KM, Renes IB, Verburg M, Buller HA, Dekker J. Gastrointestinal expression and partial cDNA cloning of murine Muc2. Am J Physiol Gastrointest Liver Physiol. 1999;276:G115–G124. doi: 10.1152/ajpgi.1999.276.1.G115. [DOI] [PubMed] [Google Scholar]
- Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- Wacklin P, Makivuokko H, Alakulppi N, Nikkila J, Tenkanen H, Rabina J, Partanen J, Aranko K, Matto J. Secretor genotype (FUT2 gene) is strongly associated with the composition of Bifidobacteria in the human intestine. PLoS One. 2011;6:e20113. doi: 10.1371/journal.pone.0020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ju T, Ding X, Xia B, Wang W, Xia L, He M, Cummings RD. Cosmc is an essential chaperone for correct protein O-glycosylation. Proc Natl Acad Sci USA. 2010;107:9228–9233. doi: 10.1073/pnas.0914004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JC, Ong E, Fukuda M. Molecular cloning and expression of a novel beta-1, 6-N-acetylglucosaminyltransferase that forms core 2, core 4, and I branches. J Biol Chem. 1999;274:3215–3221. doi: 10.1074/jbc.274.5.3215. [DOI] [PubMed] [Google Scholar]