Figure 9. Physiological complementation of a B. subtilis proHJ mutant strain by the heterologous proHJAA operon of B. licheniformis DSM 13T.
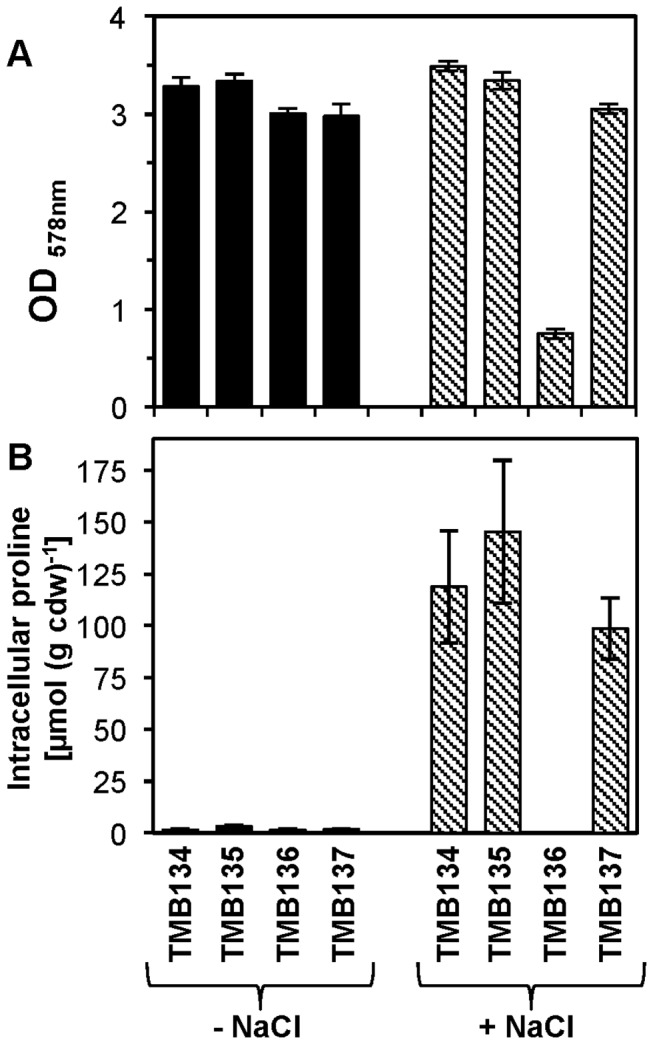
The proHJAA operon of B. licheniformis DSM 13T was cloned into plasmid pX, yielding plasmid pTMB20. pTMB20 and the empty cloning vector pX (used as a control) were recombined in a single copy into the chromosomal amyE sites of the B. subtilis wild-type strain JH642 and its [Δ(proHJ::tet)1] mutant derivative JSB8 [72]. This resulted in the construction of the following B. subtilis strains: TMB134 [proHJ wild type and amyE::pX], TMB135 [proHJ wild type and amyE::proHJAA], TMB136 [Δ(proHJ::tet)1 and amyE::pX], and TMB137 [Δ(proHJ::tet)1 and amyE::proHJAA]. (A) Cultures of these strains were grown in SMM without (black bars) or with 0.8 M NaCl (hatched bars). Their growth yields (OD578) were measured after 16 h of incubation at 37 °C. (B) Proline content of recombinant B. subtilis strains grown in SMM without (black bars) or with 0.8 M NaCl (hatched bars). When the cultures had reached mid-exponential growth phase (OD578 of about 2), the cells were harvested, their total solute pool was extracted and the intracellular proline concentrations were determined by HPLC analysis. The error bars represent the standard deviations of the proline pools found in three independently grown cultures. The same set of strains as that shown in panel (A) was used for this experiment.
