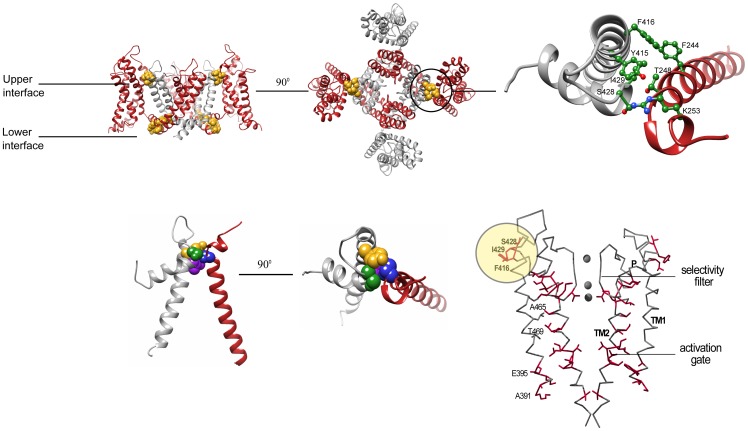
Figure 1. The upper inter-subunit domain interface as a potential communication hot spot contributing to the concerted pore opening transition.
A(Side-view ribbon representation of the Kv 1.2 structure with the S5, P and S6 labels denoting the outer, pore and inner helices, respectively, and the S1–S4 labels denoting the trans-membrane voltage-sensor helices. For clarity and since the work here involves tandem-dimer channel proteins, the two subunits are depicted in gray and red. The primary residues involved in the lower and upper intra- and inter-subunit interaction surfaces, respectively, are depicted in a space-filling gold representation. B(Top-view ribbon representation of the Kv 1.2 structure, focusing on the upper inter-subunit interaction surface. C) Zoom-in view through the upper interface. Space-filling representations of the S1 T248, S5 Y415, P S428 and P I429 residues are highlighted in blue, gold, green and purple, respectively. D) The allosteric communication trajectory of the Shaker Kv channel pore domain. Gating-sensitive positions along the pore domain of the Shaker channel, that primarily affect the late concerted pore opening, form a connected pattern when mapped onto the closed pore conformation of the KcsA K+ channel. Side-chains are shown only for gating-sensitive residues (red). Highlighted in yellow circle are the gating-sensitive pore domain residues lying at the pore side of the upper voltage-sensor-pore domain interface. Adapted from [11].