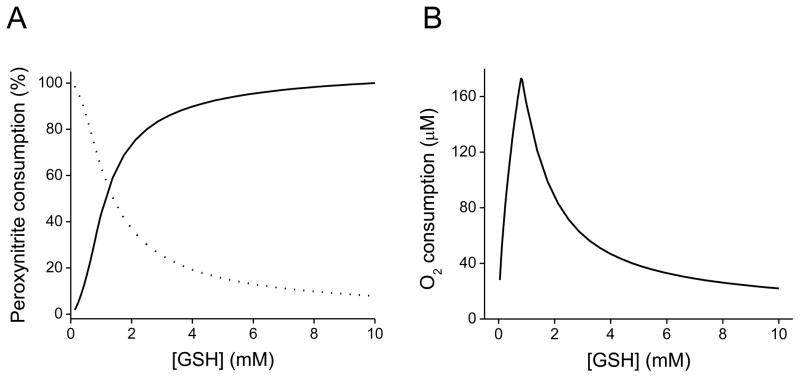
Figure 1. Computer-assisted simulations of the peroxynitrite-mediated decay in the presence of GSH.
(A) Simulated profile of peroxynitrite (0.5 mM) decay by direct reaction with GSH (0.05–10 mM) (solid line) versus homolysis to free radicals (dotted line). (B) Simulated profile of oxygen consumption against GSH concentration. The initial oxygen concentration was 0.217 mM (oxygen solubility at 37 °C). As the concentration of GSH increases, peroxynitrite decay by the direct two-electron oxidation process becomes more significant, instead of its decay through homolysis, which predominates at low GSH concentrations. Computer-assisted kinetic simulations were performed according to the reactions shown in Table 1 with the software Gepasi [161].