Figure 25.
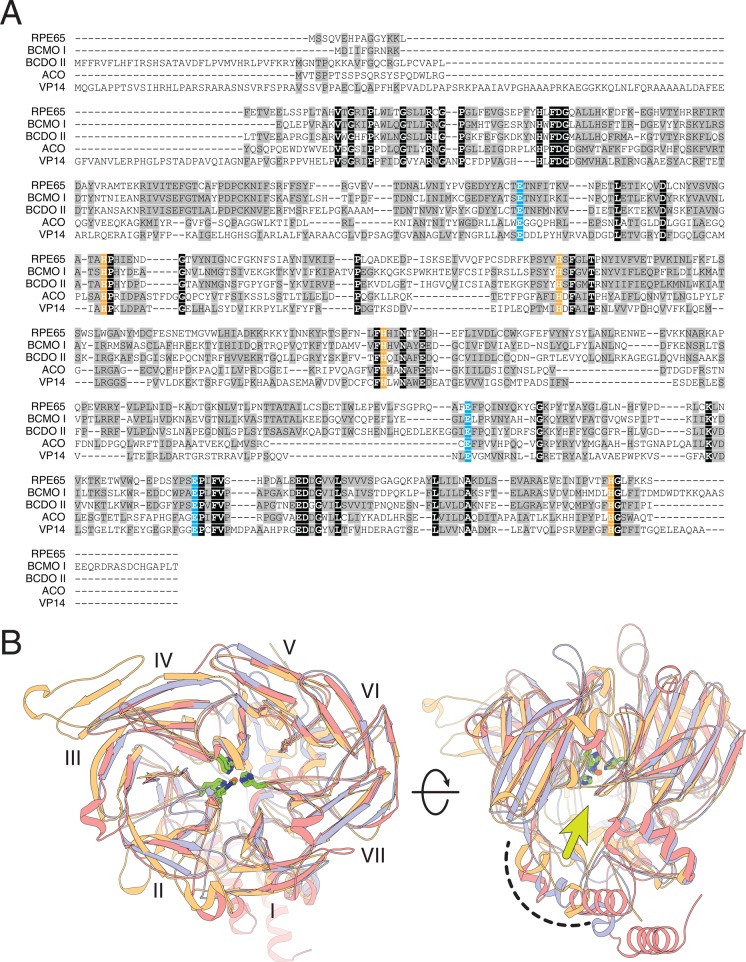
Structural alignment of CCO family members. (A) Iron-binding His residues are highlighted in orange, and second sphere Glu residues are highlighted in blue. (B) Structural superposition of CCO members of known structure (RPE65, orange; ACO, blue; VP14, pink). These enzymes adopt a 7-bladed β-propeller fold (blades labeled with Roman numerals) with a helical cap on the top face of the propeller that houses the active site and membrane-binding domain (curved, dashed line), which surrounds the active site entrance indicated by a yellow-green arrow. The iron cofactor located at the center of the propeller is coordinated by four conserved His residues (green). The two views in panel B differ by a 90° horizontal rotation.