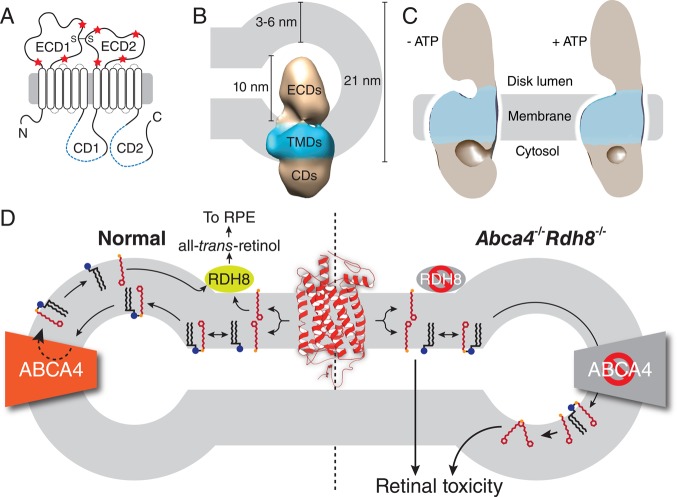
Figure 33.
Structure and function of ABCA4. (A) Two-dimensional topology diagram of ABCA4. Positions of the Walker A motifs are indicated by blue dashed lines within the CDs. Glycosylation sites are marked with red stars, and an intramolecular disulfide bridge is indicated by S–S. ECD, exocytoplasmic domain; CD, cytoplasmic domain. (B) Electron microscopic structure of ABCA4 and its dimensions relative to a ROS disk rim. TMDs, transmembrane domains. (C) Structural differences in ABCA4 in the absence and presence of ATP. (D) Role of ABCA4 in the visual cycle and pathology of elevated all-trans-retinal. ABCA4 flips the all-trans-retinal–PE complex, a product of all-trans-retinal (red line structure) condensation with PE (black line structure with blue sphere indicating the headgroup), to the outer leaflet of the disk membrane, allowing dissociation of the complex and subsequent reduction of all-trans-retinal to all-trans-retinol, which then reenters the visual cycle (left). Retinal pathology is observed in mice lacking ABCA4 and RDH8 activities due to accumulation of all-trans-retinal and its lipid adducts (right).