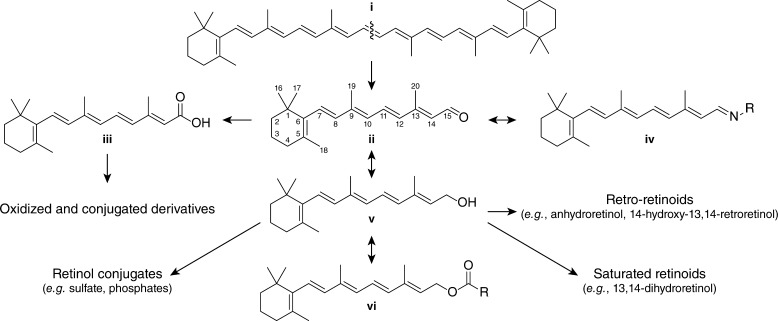
Figure 5.
Retinoid metabolism in vertebrates. Dietary all-trans-β,β-carotene (i), obtained primarily from plants, is oxidatively cleaved in a symmetric manner by β-carotene monooxygenase I (BCMO I), yielding two molecules of all-trans-retinal (ii). Retinal can reversibly combine with an amino group to form a retinyl imine (Schiff base) (iv). Retinal is also subject to oxidation and reduction to form retinoic acid (iii) and retinol (vitamin A) v, respectively, the latter in a physiologically reversible manner. Retinoic acid can be converted into several conjugated and/or oxidized derivatives, some of which exert biological effects. Retinol also can be converted into several derivatives including retro-retinoids, saturated retinols, and phosphate conjugates. Retinol is also reversibly esterified to produce retinyl esters (vi), the main storage form of vitamin A in the body.