Table 1.
One-pot, sequential RCM/CM/chemoselective hydrogenation involving type I olefins.
| entry | olefin | yield %a (avg %) |
RCM-CM- chemoselective hydrogenation |
|---|---|---|---|
| 1 |
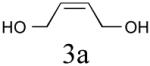
|
64%b (86%) |
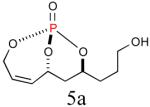
|
| 2 |
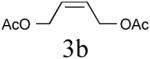
|
56% (82%) |
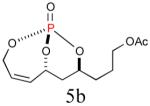
|
| 3 |
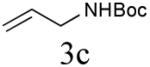
|
59%b (84%) |
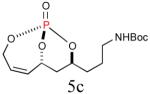
|
| 4 |
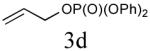
|
40% (74%) |
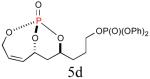
|
| 5 |
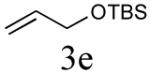
|
52% (80%) |
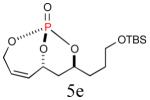
|
| 6 |
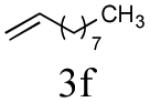
|
65% (87%) |
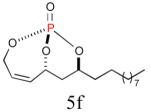
|
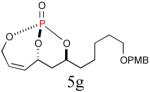
| 7 |
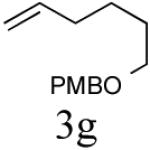
|
43%c (76%) |
|
All reactions were performed using freshly distilled (over CaH2) FDT solvents
1,4-Benzoquinone is not used during RCM event.
Reaction was performed in CH2Cl2 purified by passing through basic Al2O3 and degassed by argon purging without any additives.
