Table 2.
One-pot, sequential RCM/CM/chemoselective hydrogenation involving type II olefins
| entry | olefin | yield %a (avg %) |
RCM-CM-chemoselective hydrogenation |
|---|---|---|---|
| 1 |
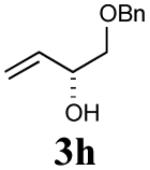
|
41% (75%) |
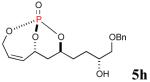
|
| 2 |
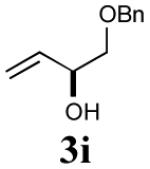
|
35% (71%) |
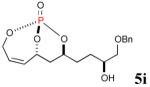
|
| 3 |
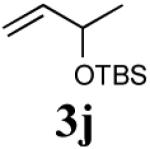
|
69% (89%) |
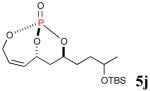
|
| 4 |
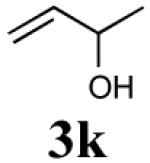
|
48% (78%) |
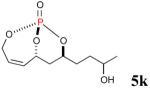
|
| 5 |
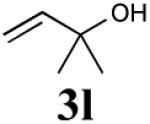
|
72% (90%) |
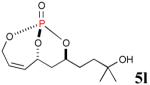
|
| 6 |
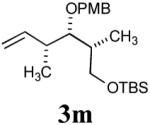
|
30%b (67%) |
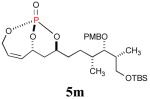
|
| 7 |
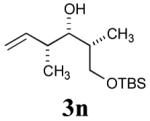
|
54% (81%) |
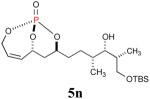
|
| 8 |
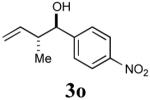
|
85% (95%) |
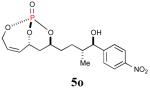
|
| 9 |
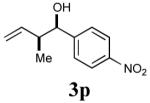
|
79% (92%) |
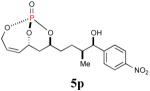
|
All reactions were performed using freshly distilled (over CaH2) FDT solvents
Reaction was performed in CH2Cl2, 1,2-DCE purified by passing through basic Al2O3 and degassed by argon purging.
