Fig. 7.
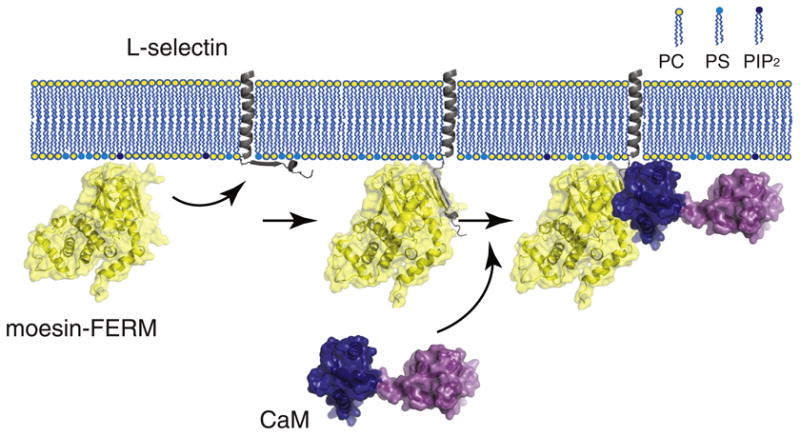
A model illustrating formation of the moesin/l-selectin/CaM ternary complex at the membrane interface. The FERM domain of moesin (moesin-FERM) is shown in the yellow-coated ribbon diagram. The transmembrane and cytoplasmic domains of l-selectin are shown in gray, with the former embedded in the membrane bilayer and the latter adhered to the anionic membrane surface. The large extracellular domain of l-selectin is not shown. CaM is shown with its two lobes (shown in dark blue and purple) separated and in an extended conformation. (Left) The FERM domain of moesin is recruited to the membrane due to its association with anionic phospholipids. Meanwhile, the cytoplasmic domain of l-selectin that is enriched in basic residues is associated with anionic membrane surface [29]. (Middle) The FERM domain of moesin binds the cytoplasmic domain of l-selectin and desorbs it from the membrane surface, as shown in this study. CaM alone does not interact with the membrane-adhered cytoplasmic domain of l-selectin [29]. (Right) Only after moesin association does CaM bind the cytoplasmic domain of l-selectin to form the ternary complex, in which CaM takes on an extended conformation [42].
