Figure 1.
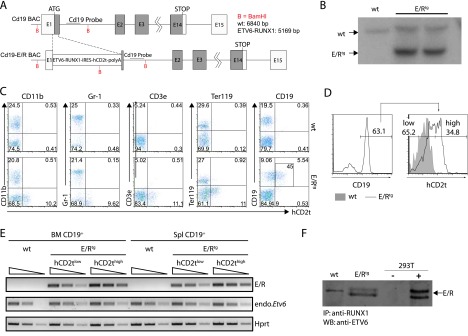
Design of BAC and expression analysis of the ETV6/RUNX1 transgene. (A) The ETV6/RUNX1-IRES-hCD2t expression cassette was inserted in the first exon of the Cd19 gene. (B) Southern blot analysis of the Cd19 locus in an ETV6/RUNX1 founder mouse. Genomic DNA was digested with BamHI, blotted on a nitrocellulose membrane, and hybridized with a radiolabeled DNA probe against the proximal region of the Cd19 intron 1. The expected insertion of the ETV6/RUNX1-IRES-hCD2t-polyA cassette gives rise to a labeled DNA fragment of 5169 bp in contrast to the wt Cd19 allele (6840 bp). (C) The reporter hCD2t is specifically expressed in CD19+ cells. BM cells of wt mice (upper panel) and E/R-expressing mice (lower panel) were isolated, stained for surface markers CD11b, Gr1, CD3e, Ter119 as well as CD19, and analyzed by flow cytometry. The reporter gene hCD2t is only expressed in CD19+ cells but not in other hematopoietic lineages. Black inlet shows percentage of hCD2t+ cells from the CD19-expressing population. (D) Distribution of the hCD2t expression levels on the entire CD19+ cell fraction. One representative histogram of hCD2thigh/low-expressing CD19+ cells is shown. (E) mRNA expression analysis for ETV6/RUNX1 and Etv6 in semiquantitative reverse transcription-PCR in FACS-sorted hCD2tlow/high cells of the BM and the spleen of wt and E/Rtg mice. The wedge shows five-fold dilution series. (F) ETV6/RUNX1 protein expression was analyzed by immunoprecipitation (anti-RUNX1) and Western blot analysis (anti-ETV6) from E/Rtg and wt fetal liver-derived B cell lines. As control, 293T cells transiently transfected with an E/R-expressing plasmid were used. An unspecific antibody cross-hybridization signal is visible in some of the samples (wt).
