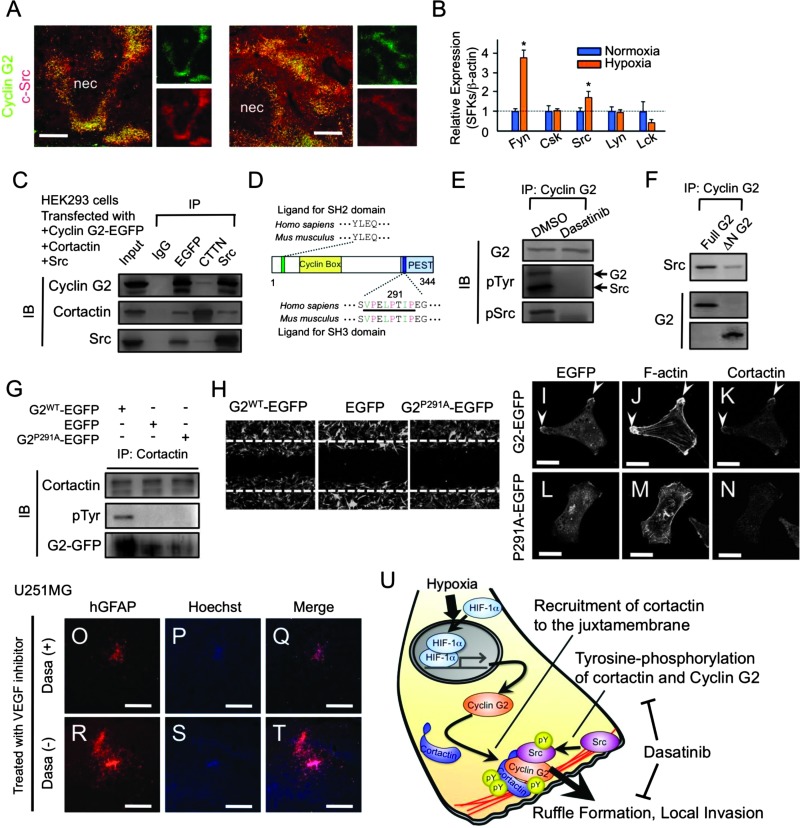
Figure 5.
Molecular mechanism of the cyclin G2-driven tyrosine phosphorylation of cortactin and consequent invasion. (A) Immunofluorescent analysis showing that c-Src is abundantly expressed in pseudopalisades and most signals overlap with signals for cyclin G2. (B) Hypoxia significantly promotes the transcription of SFKs, Fyn, and Src in U87MG cells. (C) Cyclin G2, cortactin, and SFKs are co-immunoprecipitated. (D) Schematic diagram of the cyclin G2 protein. Cyclin G2 has a putative SH2 domain-binding motif at the N terminus and a noncanonical SH3 domain-binding motif (bar; 287–294) at the C terminus. Green letters indicate aliphatic amino acids (valine, leucine, and isoleucine), and red letters indicate proline. (E) Immunoprecipitation and immunodetections in HEK293 cells transfected with cyclin G2-EGFP and Src. Cyclin G2 is tyrosine-phosphorylated in an SFK-dependent manner. (F) Deletion of the N terminus (ΔN-G2) of cyclin G2 impairs the interaction with Src. (G and H) A single amino acid mutation (P291A) in the SH3 domain-binding motif of cyclin G2 (G2P291A) impaired the induction of cortactin tyrosine phosphorylation (G) and consequent cell migration (H). (I–N) The P291A mutant impairs recruitment of cortactin to juxtamembrane. (O–T) Dasatinib inhibited the GBM expansion enhanced by anti-vascular endothelial growth factor treatment. (U) A model describing the role of cyclin G2 in the hypoxia-driven local invasion of glioblastoma cells. Cyclin G2 recruits cortactin to the leading edge of migrating glioma cells and promotes the consequent tyrosine phosphorylation of cortactin, which is essential for ruffle formation and tumor invasion. The scale bars represent 200 µm (A, O–T); “nec” indicates the necrotic foci in GBM specimens.