Abstract
A novel glassy carbon electrode (GCE) modified with carbon-spheres has been fabricated through a simple casting procedure. The modified GCE displays high selectivity and excellent electrochemical catalytic activities towards dopamine (DA), serotonin (5-HT), and ascorbic acid (AA). In the co-existence system, the peak separations between AA and DA, DA and 5-HT, and AA and 5-HT are large up to 230, 180, and 410 mV, respectively. Differential pulse voltammetry (DPV) has been employed to simultaneously detect DA, 5-HT, and AA, and the linear calibration curves for DA, 5-HT, and AA are obtained in the range of 20.0–150.0 μM, 40.0–750.0 μM and 300.0–2,000.0 μM with detection limits (S/N = 3) of 2.0 μM, 0.7 μM and 0.6 μM, respectively. The proposed electrode has been applied to detect DA, 5-HT, and AA in real samples using standard addition method with satisfactory results.
Keywords: carbon-spheres, simultaneous determination, dopamine, ascorbic acid, serotonin
1. Introduction
Neurotransmitters, such as dopamine (3,4-dihydroxyphenylethylamine, DA) and serotonin (5-hydroxytryptamine, 5-HT) are the chemical messengers which transmit messages from one neuron to the next. This transmission proceeds through the secretion of neurotransmitters from one neuron and then binding to the specific receptor located on the membrane of the target cell. This interaction between neurotransmitter and receptor is one of the major modes of communication between neurons [1,2]. Dopamine is one of the most typical catecholamines and belongs to the family of inhibitory neurotransmitters. A loss of DA-containing neurons may result in some serious diseases such as schizophrenia and Parkinson's disease [3,4]. 5-HT is widely distributed in the brain, and together with other neurotransmitters, makes a significant contribution to brain functions such as sleep, thermoregulation, food intake, and sexual activity, as well as in psychopathological states such as depression, anxiety, alcoholism, and drug dependency [5,6]. Ascorbic acid (AA) has a significant role in normal neuronal physiology and acts as an important antioxidant, enzyme co-factor, and neuromodulator in the brain [7]. These three substances are all electro-active and coexist in biological systems such as brain tissue. There has been a considerable effort to develop voltammetric methods for the determination of AA, DA, and 5-HT in real biological matrixes. It is well known that the direct redox reactions of these three species at bare electrodes are irreversible and therefore require high overpotentials. Moreover, the direct redox reactions of these species at the bare electrodes take place at very similar potentials and they suffer from a pronounced fouling effect, which results in rather poor selectivity and reproducibility [8–12], so the ability to selectively detect DA, 5-HT and AA, has been a matter of great interest to bioelectrochemists, electroanalytical chemists and neuroscientists [8–12]. Up to now, there are a few reports in the literature about the simultaneous determination of DA, 5-HT and AA which used different electrochemical methods and electrodes for their simultaneous determination. For example, various chemically modified electrodes including electrodeposited nanostructured platinum on Nafion-coated GCEs [13], poly(o-phenylenediamine)- and poly(phenosafranine)-modified GCEs [14,15], choline- and acetylcholine-modified GCEs [16], DNA-immobilized carbon fibre microelectrodes [17,18], solid carbon paste electrode modified with a nonionic polymer film [19], and carbon paste electrodes modified with iron(II) phthalocyanine complexes [20] have been reported for the simultaneous determination of DA, 5-HT and AA. A proposed methodology for the discrimination between DA and 5-HT in the presence of AA with the combination of a large amplitude/ high frequency voltage excitation and signal processing techniques [21] is also reported in the literature. However, direct use of carbon materials (i.e., without other modifiers) for the simultaneous determination of these three species have rarely been reported [22–26]. To the best our knowledge, only carbon-nanotube-modified GCEs [22], carbon-nanotube-intercalated graphite electrodes [23], graphite electrodes reinforced by carbon [24] edge plane pyrolytic graphite electrode [25], and carbon nanofiber [26] have been employed as simple electroanalytical methodology for the simultaneous determination of dopamine, serotonin and ascorbic acid.
Recently, we have synthesized a new kind of micro-structured porous carbon materials, double-shelled carbon spheres (CS), and we have shown their excellent electrochemical properties as electrode materials such as good conductivity, porous nature and high specific area [27–30]. Herein, the feasibility of using a CS-modified glassy carbon electrode(CS/GCE) in an attempt to develop a sensitive voltammetric method for the simultaneous determination of DA, 5-HT and AA in pH 7.00 phosphate buffer solution is reported.
2. Experimental Section
2.1. Chemicals and Instrumentation
Ascorbic acid (AA), dopamine (DA), serotonin (5-HT) and other chemicals were all purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). All these chemicals are of analytical grade and used as received. Unless otherwise indicated, phosphate buffer (pH 7.0, 0.1 M) was used as supporting electrolyte. Phosphate buffer was prepared with KH2PO4 and Na2HPO4, and the desired pH was modulated with NaOH or H3PO4 by pH meter. Freshly prepared AA, DA and 5-HT solutions were used for each experiment. All aqueous solutions were prepared with triply-distilled water. Morphological characterization of the obtained carbon spheres was performed on a transmission electron microscope (SEM) (Hitachi S-4800, Tokyo, Japan) operating at 3 kv. A very dilute dispersion of the carbon spheres in ethanol was dispersed onto carbon-coated copper grids and the ambient dried carbon spheres were vacuum sputtered for SEM observation. All electrochemical experiments were performed on CHI760D electrochemical working station (CHI, Shanghai, China) with a conventional three-electrode cell which consists of a bare GCE or CS/GCE as the working electrode, a Ag/AgCl as the reference electrode, and a platnium wire as the counter electrode.
2.2. Preparation of CS Film Electrodes
Prior to surface modification, the glassy carbon disk electrodes (GC, 3-mm diameter, Bioanalytical System, Inc., West Lafayette, IN, USA) were first polished with 0.3 and 0.05 μm alumina slurry on a polishing cloth, respectively, and then sonicated in the acetone and distilled water for 3 min, respectively. The as-synthesized CS was dispersed into N,N-dimethylformamide (DMF) to give a homogeneous suspension (10 mg·mL−1) under sonication. A 2.0 μL aliquot of the homogeneous suspension was cast onto the GCE surface and allowed to dry under a lamp to evaporate the solvent, thus a CS-modified GCE (denoted as CS/GCE) was obtained.
3. Results and Discussion
3.1. Characterization of Synthesized Carbon Spheres
The synthesis of carbon spheres and the detailed characterization of the synthesized carbon spheres including SEM, TEM, IR spectra, elemental analysis, Raman spectra, and BET tests were reported in our previous publications [26–29]. In brief, the carbon spheres with porous shell are about 480 nm in diameter. BET specific surface area was 194 m2/g, and the total pore volume was 0.36 cm3/g. Elemental analysis shows that the carbon spheres contain 94.0 wt.% C, 1.0 wt.% H, 2.7 wt.% O and 1.0 wt.% S, suggesting that oxygen-containing functional groups are present on the CS surface. The SEM image of the synthesized carbon sphere is shown in Figure 1.
Figure 1.
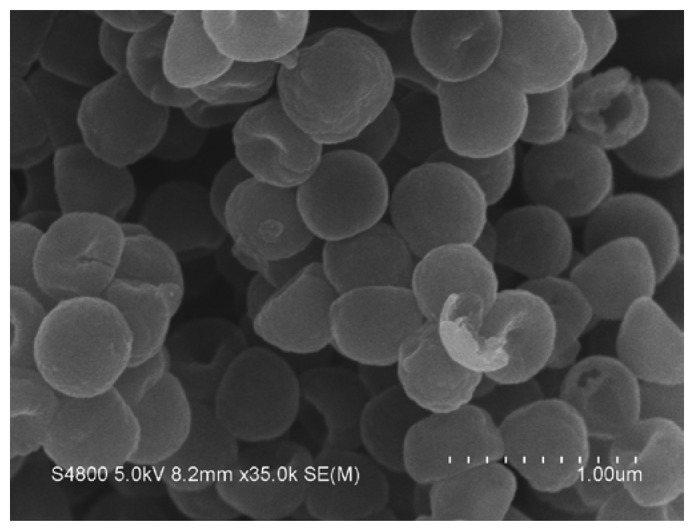
SEM image of the synthesized carbon spheres.
3.2. Electrochemical Responses of AA, DA and 5-HT on CS/GCE
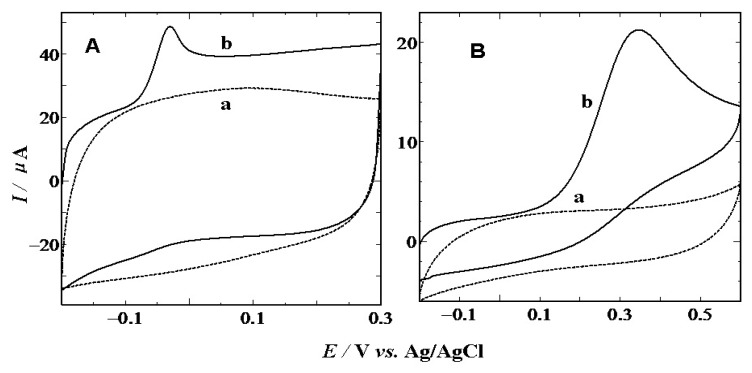
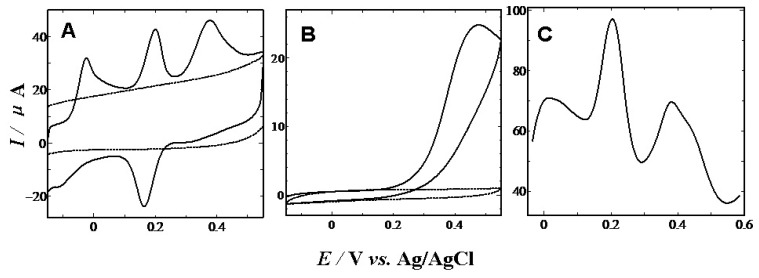
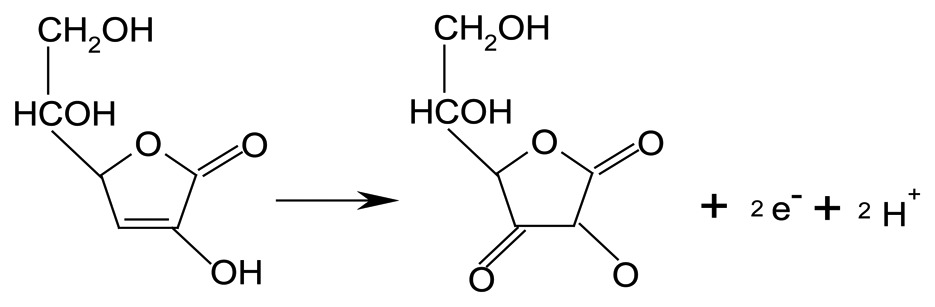
Figure 2 depicts cyclic voltammograms (CVs) of the electro-oxidation of 1 mM AA at the CS modified (A) and bare (B) GCEs in 0.1 mol·L−1 PBS (pH 7.0) with a scan rate of 100 mV·s−1, respectively. At a bare GCE, AA shows a broad and irreversible oxidation peak at 0.35 V, while the oxidation peak shifted to −0.03 V with well-defined peak shape at the CS/GCE. The 380 mV negative shift of the anodic peak indicates that the CS modified GCE also plays a strong catalytic effect on the AA electro-oxidation.
Figure 2.
Cyclic voltammograms obtained at CS/GCE (A), and bare GCE (B) in the presence(solid line, curve b) or absence(dotted line, curve a) of 1 mM of AA in the phosphate buffer solution (pH 7.0) with a scan rate of 100 mV·s−1.
In the electrooxidation process of AA, ascorbate is oxidized to dehydroascorbate accompanied by with transfer of 2-electrons and 2-protons, and the electrochemical reaction can be expressed as follows:
 |
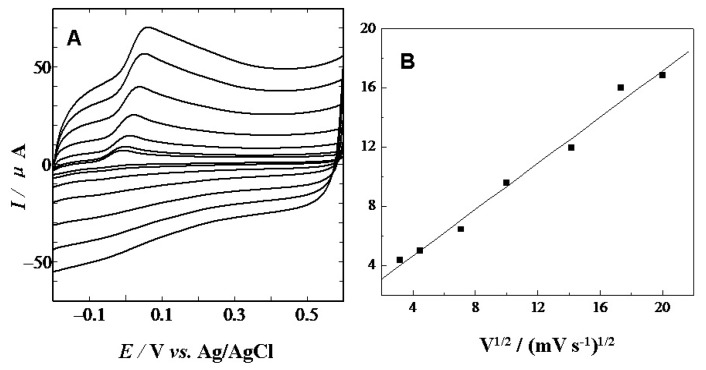
As shown in Figure 3, the anodic peak currents were proportional to the square roots of scan rates in the range of 10–400 m·Vs−1, indicating that the electrochemical reactions of AA at CS/GCE is a diffusion-controlled process.
Figure 3.
(A) CVs obtained at CS/GCE of 1 mM of AA in the phosphate buffer solution (pH 7.0) at different scan rates (from inner to outer): 10, 20, 50, 100, 200, 300, 400 mV·s−1; (B) The plot of currents against the square roots of scan rates.
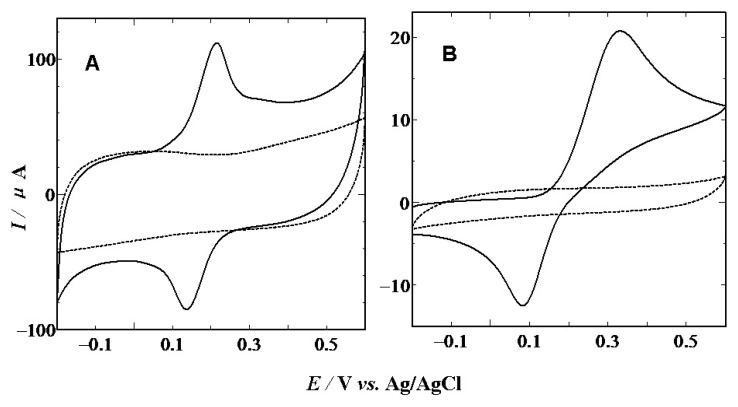
Figure 4 shows the cyclic voltammmograms of 1 mM DA at CS modified (A) and bare (B) GCEs in the phosphate buffer solution (pH 7.0) with a scan rate of 100 mV·s−1, respectively. As can be seen, DA shows a sluggish and much smaller CV peak response with a ΔEp of 0.35 V at bare GCE (B). But at the CS modified GCE, the peak potential shifted negatively and showed a pair of quasi-reversible redox peaks with a ΔEp of only 90 mV. The decreased peak separation strongly indicates excellent catalytic activity of CS to the electro-oxidation of DA. The electrochemical process can be expressed by the following equation:
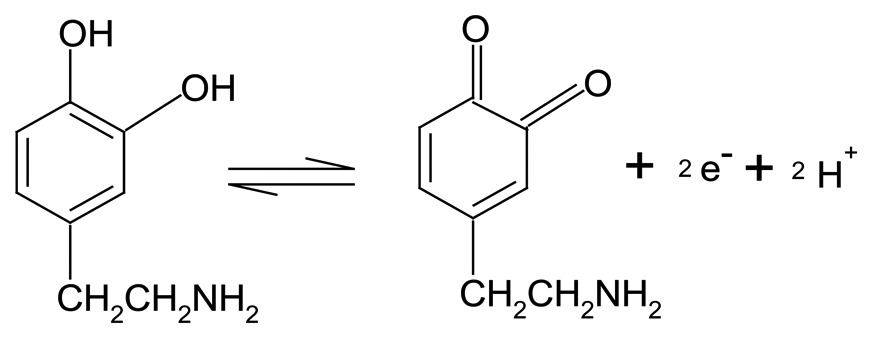 |
Figure 4.
CVs obtained at CS/GCE (A), and bare GCE (B) in the presence (solid line) or absence (dotted line) of 1 mM of DA in the phosphate buffer solution (pH 7.0) with a scan rate of 100 m·Vs−1.
Figure 5 shows the CVs of 5-HT at CS modified (A) and bare (B) GCE, respectively. The voltammetric peak of 5-HT in the neutral pH 7.0 PBS appeared at about 0.46 V at the bare GC electrode (B) and the peak was rather broad, indicating a slow electron transfer kinetic. However, a sharp oxidation peak at 0.38 V and a small re-reduction peak at 0.3 V were obtained at CS/GC electrode (A). The 80 mV negative shift indicates a catalytic effect of the CS modified layer on the electro-oxidation of 5-HT. It must be pointed out that the appearance of the re-reduction peak of 5-HT oxidation indicates that reaction reversibility increases and the rate of following reactions at the CS/GCE reduces. The electrochemical process can be expressed as the following equation:
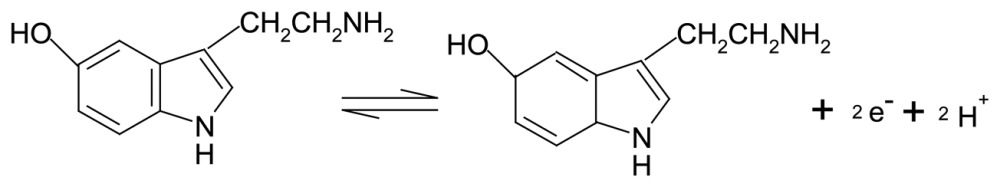 |
Figure 5.
CVs obtained at CS/GCE (A), and bare GCE (B) electrode in the presence(solid line) or absence(dotted line) of 1.5 mM of 5-HT in the phosphate buffer solution (pH 7.0) with a scan rate of 100 m·Vs−1.
For the oxidation of DA and 5-HT, the anodic peak currents of DA and 5-HT are also both proportional to the scan rates and displayed a diffusion-controlled electrode reaction (data not shown).
Comparing with the bare electrode, the porous interfacial layer of the CS-modified electrode with a high specific surface area increases the conductive area, biomolecules can penetrate through the conductive porous channels onto the electrode more easily [23], leading to higher sensitivity and selectivity. On the other hand, edge plane of CS with its large number of edge plane sites facilitates and accelerates the electron-transfer rate between species and electrode [25] and therefore results in sensitive, well-defined and resolved signals for DA, 5-HT and AA, providing an electroanalytical method for the determination of DA, 5-HT and AA.
3.3. Simultaneous Determination of DA, 5-HT and AA at CS/GCE
Since DA, 5-HT and AA have similar oxidation potentials at most solid electrodes, separate determination of these species is a great problem due to their overlapping signals. In order to establish a sensitive and selective method for the quantification of DA, 5-HT and AA, the electrochemical oxidation of the mixture containing these three species at the CS modified GCE was studied. As shown in Figure 6B, the CV of the mixture solution containing DA, 5-HT and AA shows broad and overlapped anodic peaks at bare GCE, so the peak potentials for DA, 5-HT and AA are indistinguishable at a bare GCE and therefore, it is impossible to deduce any information from the broad and overlapped voltammetric peak. However, at the CS/GCE, the overlapped voltammetric peak is resolved into three well-defined anodic peaks at about −0.03, 0.20 and 0.38 V (A), corresponding to the oxidation of AA, DA and 5-HT, respectively, which are also observed in DPV mode (C). The separations of peaks were 230 mV, 180 mV and 410 mV between DA and AA, DA and 5-HT, and AA and 5-HT, respectively, which were large enough to determine DA, 5-HT and AA individually and simultaneously.
Figure 6.
Cyclic voltammetry recordings of 2 mM AA, 0.5 mM DA and 0.5 mM HT at CS/GCE (A) and bare GCE (B) in 0.1 M phosphate buffer solution (pH 7.0) in the presence (solid line) and absence (dotted line) of AA, DA and HT with a scan rate of 100 mV·s−1; (C) Differential pulse voltammograms of the same mixture at CS/GCE.
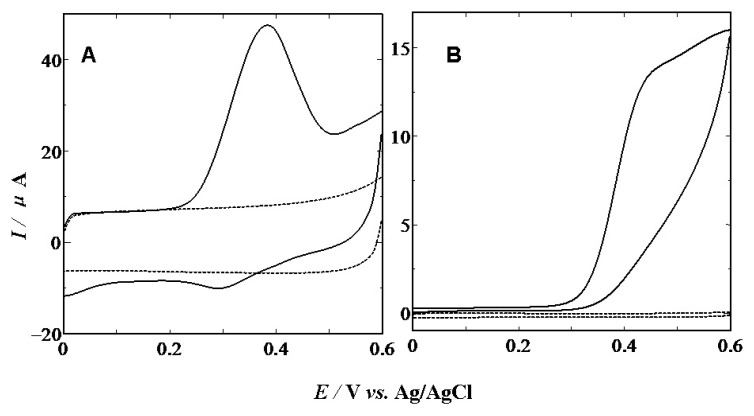
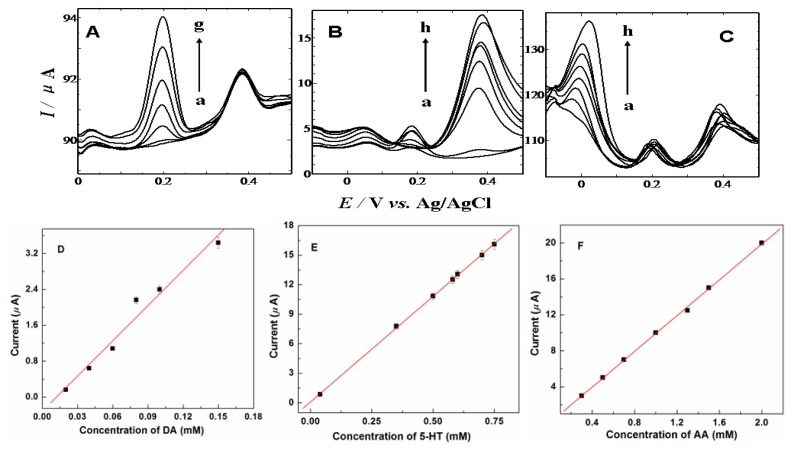
The electro-oxidation processes of DA, AA and 5-HT in the mixture have also been investigated when the concentration of one species changed, whereas those of other two species are kept constant. DPV was employed to detect AA, DA and 5-HT because of its higher current sensitivity and better resolution than CV technique. Figure 7A gives the DPV recordings at various DA concentrations at the PVA modified GCE in the presence of AA and 5-HT. From Figure 7A, it can be seen that the peak current of DA increased with an increase in DA concentration when the concentrations of AA and 5-HT were kept constant. Similarly and obviously, as shown in Figure 7B,C, keeping the concentrations of other two compounds constant, the oxidation peak current of 5-HT and AA was positively proportional to its concentration, while those of other two compounds did not change. From our experimental results depicted above, it can be obtained that the electrochemical response peaks for DA, AA and 5-HT oxidation at the PVA modified GCE were clearly separated from each other when they co-exist in pH 7.0 PBS. It is therefore possible to simultaneously determine DA, AA and 5-HT in samples at a CS modified GCE. Under the optimum conditions, using the DPV mode, the catalytic current peak was linearly related to DA, AA and 5-HT concentration. The analytical parameters for the simultaneous determination of DA, 5-HT and AA are listed in Table 1.
Figure 7.
(A) DPVs of various concentrations of DA in 1 mM AA and 40 μM 5-HT solution (from a to g: 0, 0.02, 0.04, 0.06, 0.08, 0.1, 0.15 mM); (B) DPVs of various concentrations of HT in 1 mM AA and 12 μM DA solution (from a to h: 0, 0.04, 0.35, 0.5, 0.58, 0.6, 0.7, 0.75 mM); (C) DPVs of various concentrations of AA in 12 μM DA and 40 μM 5-HT solution (from a to h: 0, 0.3, 0.5, 0.7, 1.0, 1.3,1.5, 2.0 mM); (D)–(F) are the linear plots of currents against concentrations of DA, 5-HT, and AA, respectively.
Table 1.
Analytical parameters for the determination of DA, 5-HT, and AA.
| Analyte | Linear Range (mM) | Linear Regression Equation (ΔI: μA; C: mM) | Correlation Coefficient (r) | Detection Limit (μM) |
|---|---|---|---|---|
| DA | 0.02–0.15 | ΔI = −0.32 + 26.18 C | 0.985 | 0.2 |
| 5-HT | 0.04–0.75 | ΔI = 0.09 + 21.44 C | 0.999 | 0.7 |
| AA | 0.3–2.0 | ΔI = −0.01 + 9.94 C | 0.999 | 0.6 |
3.4. Interferences, Stability and Repeatability
To evaluate the selectivity, we investigated the current response of CS/GCE to the ternary mixture containing 40 μM DA, 40 μM 5-HT, and 1 mM AA in the presence of different interfering substances. The effects of main relevant metal ions such as K+, Na+, Mg2+, Zn2+, Fe2+, Fe3+, Cl−, NO3−, SO42− on the current of the modified electrode for the mixture were studied and the results showed that the 1,000-fold excesses of Na+, K+, Mg2+, Zn2+, Cl−, NO3−, PO43−, SO42−, AC−, 700-fold excesses of Fe2+, 400-fold excesses of Fe3+, 10-fold excess of DOPAC, 5-fold excess of uric acid and 200-fold excess of oxalate, and 500-fold excess of glucose, L-cysteine, glutathione, folic acid, levodopa induced less than ±5% interference with the detection of DA, 5-HT, and AA. These results suggest that the CS/GC electrode possessed a good selective electrochemical response toward DA, 5-HT, and AA, and also indicated that CS/GC electrode could be used for the determination of DA, 5-HT, and AA in real samples.
The storage stability of CS/GCE was investigated by monitoring its current response to the ternary mixture containing 40 μM DA, 40 μM 5-HT, and 1 mM AA. The response of the electrode lost approximately 6.3%, 5.2%, and 4.8% of its original response for DA, 5-HT, and AA, respectively, after the storage of two weeks. The operational stability of the proposed electrode was also carried out by continuous assays by DPV of the ternary mixture containing 40 μM DA, 40 μM 5-HT, and 1 mM AA using one same CS/GCE in 2 h. A current decrease of ca. 5.3%, 6.2%, and 5.4% for DA, 5-HT, and AA was observed, respectively. The repeatability of the CS/GCE was also evaluated by measurements the same ternary mixture as above and the relative standard deviation (R.S.D.) for seven repeated measurements was found to be ca. 7.1%, 6.2%, and 6.4% for DA, 5-HT, and AA, respectively, suggesting that that CS/GCE is not subject to surface fouling by the oxidation products and is reproducible. Obviously, the proposed electrode shows a high stability and good reproducibility and anti-interference ability. Since the electrode fabrication is very easy and low cost, the present CS modified electrode seems to be of great utility for making electrochemical sensor for the detection of these three neurotransmitters.
3.5. Sample Analysis
The CS/GCE was applied to the amperometric assay of DA in dopamine hydrochloride injection (labeled as 10 mg·mL−1), AA in vitamin C injection (labeled as 200 mg·mL−1), and DA, 5-HT, AA in synthesized samples, respectively, by standard addition method. The injection solutions were diluted with PBS by 1,000 times and a ternary mixture containing 500 μM AA, 100 μM DA, and 100 μM 5-HT was used as synthesized samples. The results of the above assaying are listed in Table 2. The recoveries for the determinations vary from 96.5% to 105.5%, suggesting the proposed CS/GCE can be used to reliably determine DA, 5-HT and AA in real samples.
Table 2.
Results of the determination of DA, 5-HT, and AA in real samples.
| Sample |
Labeled (mg·mL−1) |
Added (mg·mL−1) |
Found (mg·mL−1) |
R.S.D (%) |
Recovery (%) |
|---|---|---|---|---|---|
| vitamin C injection | 200 | 0 | 194.2 | 1.9 | - |
| 20 | 221.1 | 2.3 | 105.5 | ||
| dopamine hydrochloride injection | 10 | 0 | 10.1 | 3.1 | - |
| 5 | 15.2 | 4.2 | 104.0 | ||
| labeled (μM) |
added (μM) |
found (μM) |
R.S.D (%) |
recovery (%) |
|
|
| |||||
| synthesized sample | AA: 500 | 0 | DA: 98.1 | 3.8 | - |
| DA: 100 | DA: 20 | 119.3 | 4.7 | 96.5 | |
| 5-HT: 100 | |||||
| AA: 500 | 0 | 5-HT: 100.5 | 4.1 | - | |
| DA: 100 | 5-HT: 20 | 120.3 | 3.9 | 101.5 | |
| 5-HT: 100 | |||||
| AA: 500 | 0 | AA: 497.9 | 3.4 | - | |
| DA: 100 | AA:20 | 519.7 | 4.9 | 98.5 | |
| 5-HT: 100 | |||||
4. Conclusions
A novel carbon spheres-modified glassy carbon electrode was fabricated. The modified electrode shows good electrocatalytic activity for the electro-oxidation of DA, 5-HT and AA. Moreover, a better separation of oxidation peaks of DA, 5-HT and AA can be achieved, indicating that the carbon spheres-modified GCE facilitates the simultaneous determination of DA, 5-HT and AA with good stability, sensitivity and selectivity. The proposed method can be applied to the determination of DA, 5-HT and AA in real samples with satisfactory results.
Acknowledgments
We are grateful for the financial support from the Natural Science Foundation of China (Grant No. 21175002), Program for New Century Excellent Talents in University (NCET-12-0599), Anhui Provincial Natural Science Foundation for Distinguished Youth (Grant No. 1108085J09), and the project sponsored by SRF for ROCS, SEM. We are also grateful for the financial support from Fuyang Teachers College (Grant No. 2012HJJC03).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Michael D.J., Wightman R.M. Electrochemical monitoring of biogenic amine neurotransmission in real time. J. Pharm. Biomed. Anal. 1999;19:33–46. doi: 10.1016/s0731-7085(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 2.Cooper J.R., Bloom F.E., Roth R.H. The Biochemical Basis of Neuropharmacology. Oxford University Press; Oxford, UK: 1982. [Google Scholar]
- 3.Wightman R.M., May L.J., Michael A.C. Detection of dopamine dynamics in the brain. Anal. Chem. 1998;70:769A–779A. doi: 10.1021/ac00164a001. [DOI] [PubMed] [Google Scholar]
- 4.Mo J.W., Ogorevc B. Simultaneous measurement of dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly (1,2-phenylenediamine)-coated carbon fiber. Anal. Chem. 2001;73:1196–1202. doi: 10.1021/ac0010882. [DOI] [PubMed] [Google Scholar]
- 5.Gershon M.D. The Second Brain. Harper Collins; New York, NY, USA: 1998. [Google Scholar]
- 6.Kema I.P., Meijer W.G., Meiborg G., Ooms B., Willemse P.H.B., de Vries E.G.E. Profiling of tryptophan-related plasma indoles in patients with carcinoid tumors by automated, on-line, solid-phase extraction and hplc with fluorescence detection. Clin. Chem. 2001;47:1811–1820. [PubMed] [Google Scholar]
- 7.Rice M.E. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- 8.Adams R.N. Probing brain chemistry with electroanalytical. Anal. Chem. 1976;48:1128A–1138A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer B., Jensen N. Stabilization of the voltammetric serotonin signal by surfactants. Electrochem. Commun. 2006;8:323–328. [Google Scholar]
- 10.Atta N.F., El-Kady M.F. Novel poly(3-methylthiophene)/Pd, Pt nanoparticle sensor: Synthesis, characterization and its application to the simultaneous analysis of dopamine and ascorbic acid in biological fluids. Sens. Actuators B: Chem. 2010;145:299–310. [Google Scholar]
- 11.Perry M., Li Q., Kennedy R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stamford J.A., Justice J.B. Probing brain chemistry: Voltammetry comes of age. Anal. Chem. 1996;69:359A–363A. [PubMed] [Google Scholar]
- 13.Selvaraju T., Ramaraj R. Electrochemically deposited nanostructured platinum on Nafion coated electrode for sensor applications. J. Electroanal. Chem. 2005;585:290–300. [Google Scholar]
- 14.Selvaraju T., Ramaraj R. Simultaneous determination of ascorbic acid, dopamine and serotonin at poly(phenosafranine) modified electrode. Electrochem. Comm. 2003;5:667–671. [Google Scholar]
- 15.Selvaraju T., Ramaraj R. Simultaneous determination of dopamine and serotonin in the presence of ascorbic acid and uric acid at poly(o-phenylenediamine) modified electrode. J. Appl. Electrochem. 2003;33:759–762. [Google Scholar]
- 16.Jin G.P., Lin X.Q., Gong J.M. Novel choline and acetylcholine modified glassy carbon electrodes for simultaneous determination of dopamine, serotonin and ascorbic acid. J. Electroanal. Chem. 2004;569:135–142. [Google Scholar]
- 17.Jiang X., Lin X. Overoxidized polypyrrole film directed DNA immobilization for construction of electrochemical micro-biosensors and simultaneous determination of serotonin and dopamine. Anal. Chim. Acta. 2005;537:145–151. [Google Scholar]
- 18.Lu L., Wang S., Lin X. Attachment of DNA to the carbon fiber microelectrode via gold nanoparticles for simultaneous determination of dopamine and serotonin. Anal. Sci. 2004;20:1131–1136. doi: 10.2116/analsci.20.1131. [DOI] [PubMed] [Google Scholar]
- 19.Wei J., He J.B., Cao S.Q., Zhu Y.W., Wang Y., Hang G.P. Enhanced sensing of ascorbic acid, dopamine and serotonin at solid carbon paste electrode with a nonionic polymer film. Talanta. 2010;83:190–196. doi: 10.1016/j.talanta.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Oni J., Nyokong T. Simultaneous voltammetric determination of dopamine and serotonin on carbon paste electrodes modified with iron(II) phthalocyanine complexes. Anal. Chim. Acta. 2001;434:9–21. [Google Scholar]
- 21.Anastassiou C.A., Patel B.A., Arundell M., Yeoman M.S., Parker K.H., O'Hare D. Subsecond voltammetric separation between dopamine and serotonin in the presence of ascorbate. Anal. Chem. 2006;78:6990–6998. doi: 10.1021/ac061002q. [DOI] [PubMed] [Google Scholar]
- 22.Wu K., Fei J., Hu S. Simultaneous determination of dopamine and serotonin on a glassy carbon electrode coated with a film of carbon nanotubes. Anal. Biochem. 2003;318:100–106. doi: 10.1016/s0003-2697(03)00174-x. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z.H., Liang Q.L., Wang Y.M., Luo G.A. Carbon nanotube-intercalated graphite electrodes for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. J. Electroanal. Chem. 2003;540:129–134. [Google Scholar]
- 24.Miyazaki K., Matsumoto G., Yamada M., Yasui S., Kaneko H. Simultaneous voltammetric measurement of nitrite ion, dopamine, serotonin with ascorbic acid on the GRC electrode. Electrochim. Acta. 1999;44:3809–3820. [Google Scholar]
- 25.Kachoosangi R.B., Compton R.G. A simple electroanalytical methodology for the simultaneous determination of dopamine, serotonin and ascorbic acid using an unmodified edge plane pyrolytic graphite electrode. Anal. Bioanal. Chem. 2007;387:2793–2800. doi: 10.1007/s00216-007-1129-y. [DOI] [PubMed] [Google Scholar]
- 26.Rand E., Periyakaruppan A., Tanaka Z., Zhang D.A., Marsh M.P., Andrews R.J., Lee K.H., Chen B., Meyyappan M., Koehne J.E. A carbon nanofiber based biosensor for simultaneous detection of dopamine and serotonin in the presence of ascorbic acid. Biosens. Bioelectron. 2013;42:434–438. doi: 10.1016/j.bios.2012.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao F., Guo X., Yin J., Zhao D., Li M., Wang L. Electrocatalytic activity of carbon spheres towards NADH oxidation at low overpotential and its applications in biosensors and biofuel cells. RSC Adv. 2011;1:1301–1309. [Google Scholar]
- 28.Zhao D., Guo X., Gao Y., Gao F. An electrochemical capacitor electrode based on porous carbon spheres hybrided with polyaniline and nanoscale ruthenium oxide. ACS Appl. Mater. Interfaces. 2012;4:5583–5589. doi: 10.1021/am301484s. [DOI] [PubMed] [Google Scholar]
- 29.Wu G., Guo X., Zhou J., Zhao D., Gao Y., Li M., Gao F. Fabrication of porous carbon spheres/manganese oxide composite electrodes for electrochemical capacitors. ECS Solid State Lett. 2012;1:M8–M12. [Google Scholar]
- 30.Wu G., Zhou J., Jiang X., Guo X., Gao F. Electrochemical detection of low-molecular-mass biothiols in biological fluids at carbon-spheres-modified glassy carbon electrodes. Electrocatalysis. 2013;4:17–23. [Google Scholar]