Figure 1.
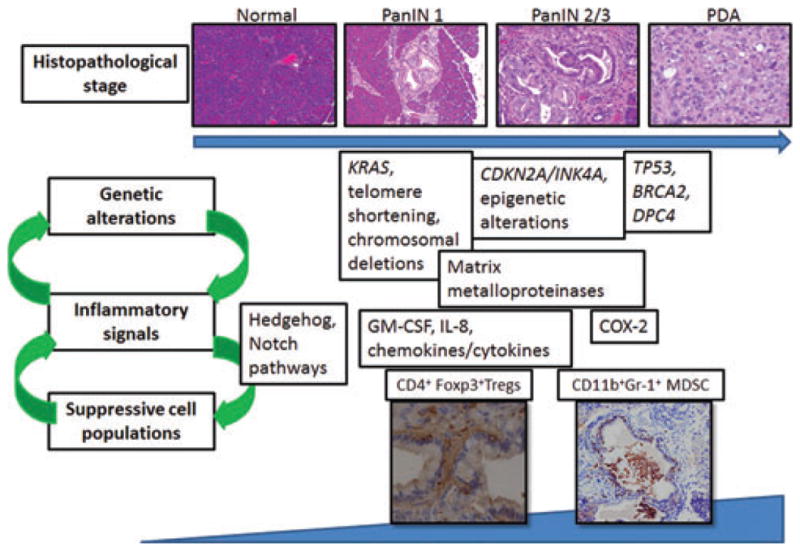
Inflammatory progression model for pancreatic ductal adenocarcinoma (PDA). Previous models have described the timing of genetic alterations in relation to different PanIN stages, which represent increasing degrees of cellular atypia, loss of normal tissue structure, and genetic abnormality. We propose a new model incorporating the relationship between genetic mutations and gene expression changes with inflammatory cytokines and signaling present in the tumor microenvironment, as well as with cell populations recruited to the tumor microenvironment. Using PDA as an example, we show that genetic mutations, such as Kras, can induce secretion of inflammatory cytokines, such as GM-CSF and IL-8, which induce and recruit immature myeloid and granulocytic cells, as well as suppressive Treg cells, that then drive immune tolerance and escape. These procarcinogenic immune cells can contribute to an inflammatory milieu, that is capable of suppressing effector T cell responses, recruiting additional suppressive cells, modifying tumor vasculature, and contributing to further DNA damage and mutations, all of which results in cancer progression.
