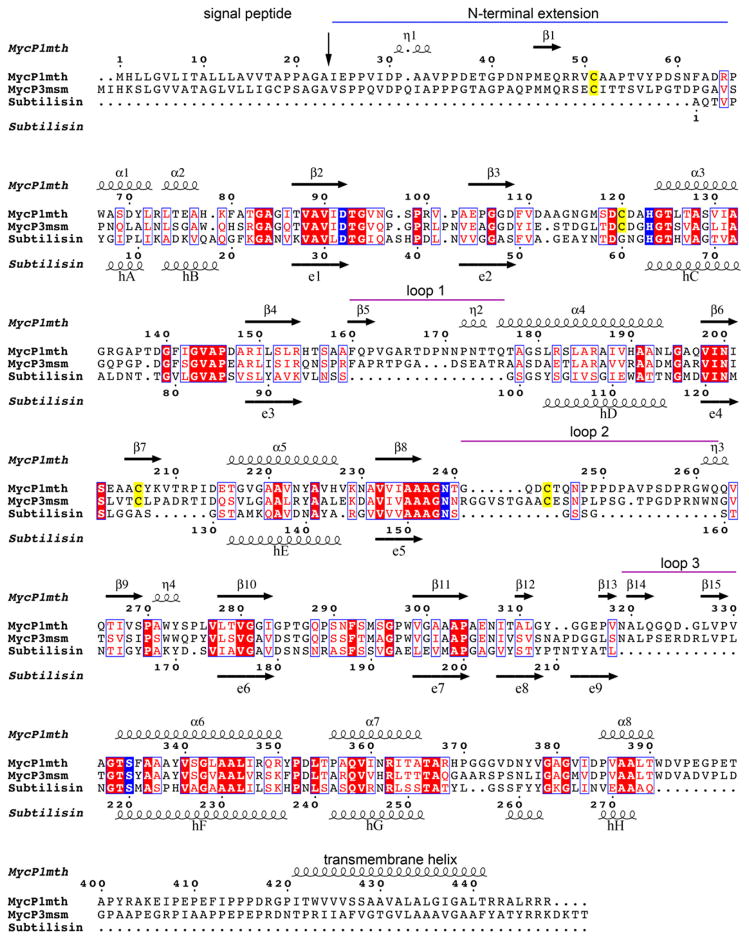
Fig. 3. Structure based sequence alignment of MycP1mth, MycP3msm and subtilisin Carlsberg.
Secondary structure elements of MycP1mth and subtilisin Carlsberg are displayed above and below the sequences, respectively. The signal protease cleavage site is indicated by vertical arrow. The N-terminal extension is indicated by a blue line. The mycosin insertion loops are indicated by purple lines. The catalytic triad residues and conserved Asn residue coordinating oxyanion hole are highlighted in blue. The conserved Cys residues that form disulfide bonds in mycosins are highlighted in yellow.