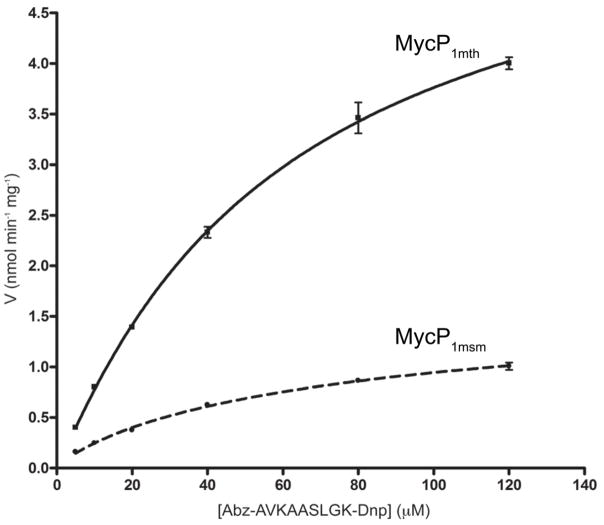
Fig. 9. Comparison of the kinetics of MycP1mth and MycP1msm in quenched fluorescent peptide assay.
Rate of substrate (Abz-AVKAA|SLGK(Dnp)-OH peptide) cleavage by MycP1mth and MycP1msm is plotted as a function of substrate concentration. Kinetic parameters were Km = 60 mM, and Vmax = 6.0 nmol min−1 mg−1 (MycP1mth) and Km = 86 mM, and Vmax = 1.78 nmol min−1 mg−1 (MycP1msm).