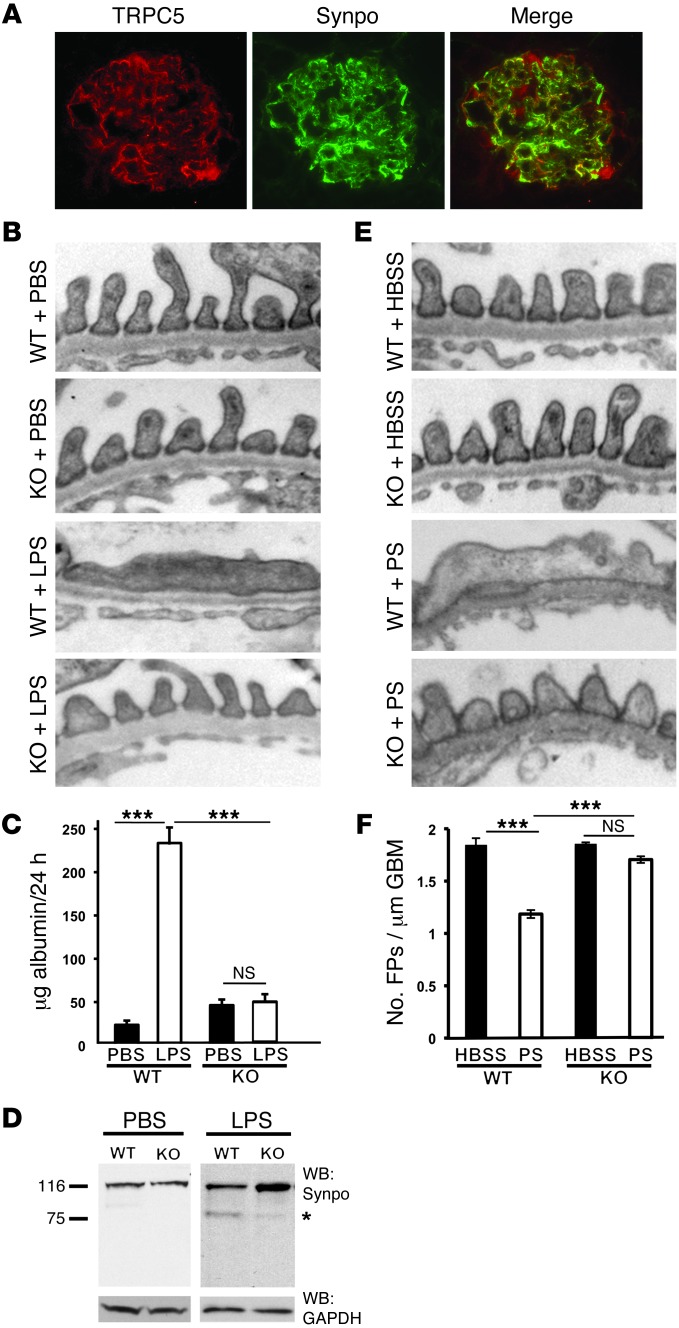
Figure 1. Genetic Trpc5 deletion is protective in 2 models of filter barrier damage.
(A) TRPC5 colocalized with synaptopodin. (B) TEM showed that WT and Trpc5-KO mice had an intact filtration barrier, demonstrated by normally arranged FPs and intervening slit diaphragms. After LPS injection, WT mice developed characteristic FPE, while Trpc5-KO mice maintained an intact filtration barrier. (C) PBS did not induce albuminuria in WT or Trpc5-KO mice. WT mice developed significant albuminuria after LPS injection, whereas Trpc5-KO mice were protected from LPS-induced albuminuria (n = 8–12 per group). (D) Western blot from isolated mouse glomeruli showed intact synaptopodin (Synpo) abundance in PBS-injected animals. LPS-injected WT mice showed synaptopodin degradation, including the appearance of the canonical 75-kDa degradation fragment (asterisk). In contrast, Trpc5-KO glomeruli were protected from synaptopodin degradation. GAPDH served as loading control. (E) WT and Trpc5-KO mice perfused with HBSS had a normal filtration barrier. Whereas WT mice perfused with PS developed FPE, Trpc5-KO mice were protected from PS-induced FPE. (F) HBSS-perfused WT and Trpc5-KO animals showed comparable FP numbers (1.8 ± 0.08 and 1.8 ± 0.01, respectively). In contrast, PS perfusion caused significant FPE in WT mice, whereas Trpc5-KO mice were protected to near-normal FP numbers (1.2 ± 0.08 and 1.7 ± 0.07, respectively; n = 6 mice and 90–150 images per group). Original magnification, ×400 (A), ×15,000 (B and E). ***P < 0.001, ANOVA.