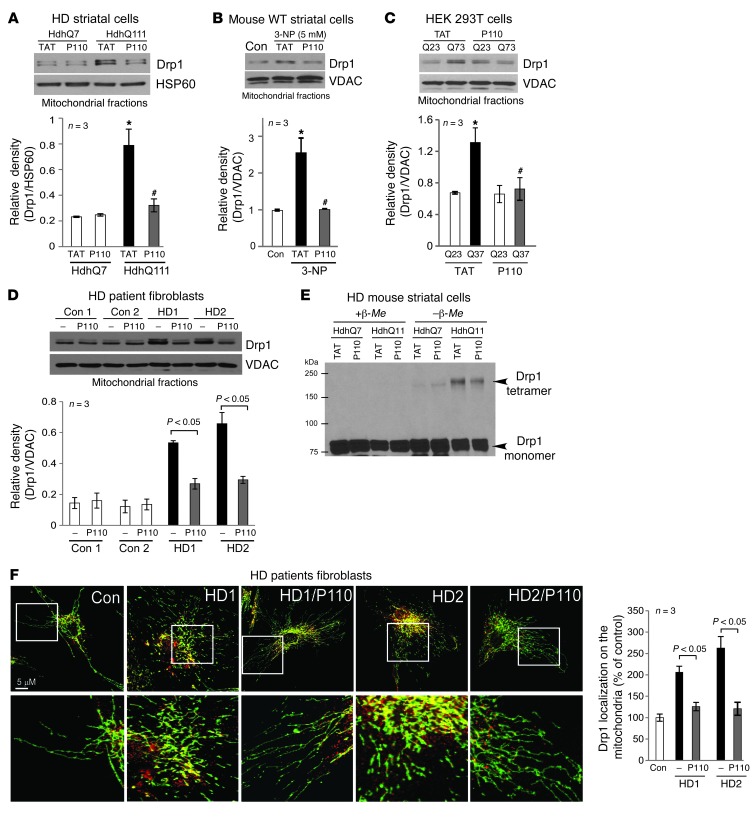
Figure 1. Treatment with P110-TAT blocked Drp1 association with the mitochondria in HD cell cultures.
(A–D) Drp1 association with the mitochondria and the effect of peptide treatment on this association was determined in 4 culture models of HD: (A) cultured mouse striatal cells expressing normal (HdhQ7) and mtHtt protein (HdhQ111) treated with peptide P110-TAT (P110, 1 μM) for 3 days; (B) mouse wild-type striatal cells incubated with P110-TAT (1 μM) for 30 minutes prior to 4-hour 3-NP exposure; (C) HEK293T cells transfected with Htt containing 23Q and 73Q repeats, followed by 2-day treatment with P110-TAT (1 μM); and (D) HD patient fibroblasts and normal fibroblasts treated with P110-TAT (1 μM) for 3 days. Western blot analysis of mitochondrial fractions was determined with anti-Drp1 antibodies (loading controls, VDAC and HSP60). Data are mean ± SEM, 3 independent experiments. (E) Total lysates of HD striatal cells were subjected to Western blot analysis using reducing or non-reducing gels and monomeric (~75-kDa) and tetrameric (~200-kDa) Drp1 levels determined; representative of 3 independent experiments. (F) Confocal microscopy of stained normal and HD patient fibroblasts with anti-Drp1 (1:250) and anti-Tom20 (mitochondria marker; 1:500). Bottom panels show enlargement of the boxed areas. Supplemental Figure 2A provides images of multiple cells. Drp1/Tom20 colocalization was determined using confocal microscopy (Pearson’s co-efficiency); data are mean ± SEM, 3 independent experiments. At least 100 cells/group were counted by an observer blinded to experimental conditions. *P < 0.05 vs. control cells; #P < 0.05 vs. cells treated with TAT.