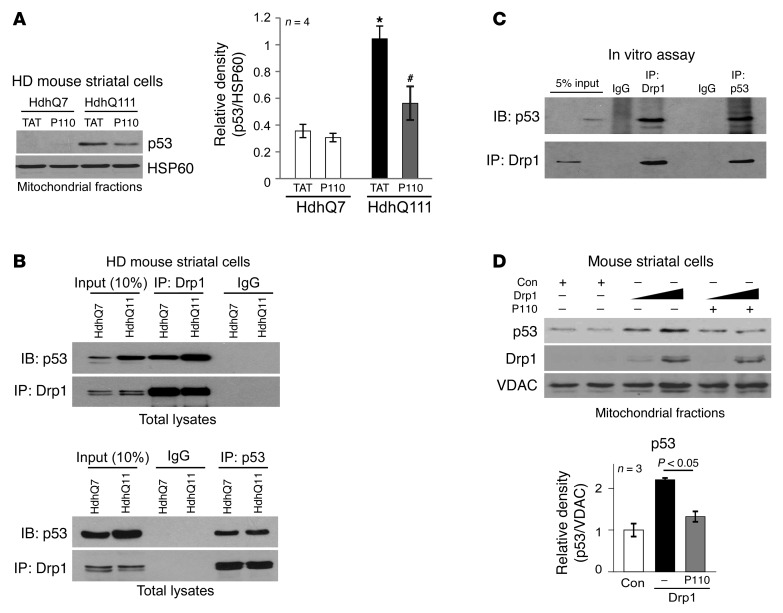
Figure 3. Drp1 bound to p53.
(A) HD striatal cells were treated with P110-TAT or control peptide TAT (1 μM each) for 3 days, and p53 association with the mitochondria was determined by Western blot analysis (loading control, HSP60). Histogram: the data represent mean ± SEM of 3–4 independent experiments. *P < 0.05 vs. wild-type cell treated with TAT; #P < 0.05 vs. HD striatal cells treated with TAT. (B) Top: Total lysates of HD striatal cells were subjected to immunoprecipitation with anti-Drp1 antibody, and immunoprecipitates were analyzed by immunoblotting with anti-p53 and anti-Drp1 antibodies. Data are representative of 3 independent experiments. Bottom: Immunoprecipitates obtained using anti-p53 antibodies were analyzed by immunoblotting using anti-Drp1 and anti-p53 antibodies. Data are representative of 2 independent experiments. (C) GST-Drp1 recombinant protein (500 ng) was incubated with p53 recombinant protein (500 ng). Immunoprecipitates with anti-Drp1 antibodies or anti-p53 antibodies were analyzed by immunoblotting with the indicated antibodies. Data are representative of 2 independent experiments. (D) Mouse wild-type striatal cells were transfected with either control vector or Drp1 plasmid (5 μg and 10 μg, respectively) for 36 hours. In the presence or absence of P110-TAT (1 μM), protein levels of p53 and Drp1 on the mitochondria were analyzed by Western blotting (loading control, VDAC). Quantification of p53 mitochondrial level in cells with Drp1 (10 μg) is presented as mean SEM of 3 independent experiments in the histogram.