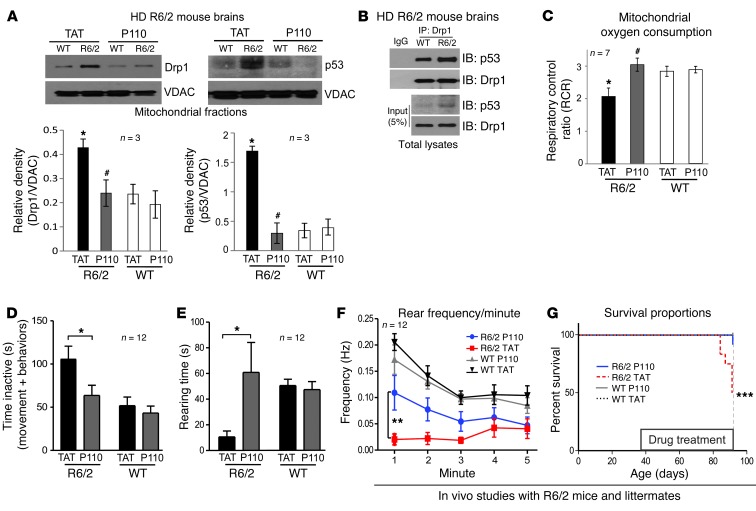
Figure 7. P110-TAT treatment reduced neurological defects and improved mitochondria function in HD R6/2 mice.
(A) Mitochondria were isolated from brains of R6/2 mice after 8 weeks of TAT or P110-TAT treatment (3 mg/kg/day). Drp1 and p53 levels in the mitochondrial fraction were determined using Western blot analysis (loading control, VDAC). Data represent 3 mice. (B) Immunoprecipitates obtained from HD R6/2 mice brains using anti-Drp1 antibodies were analyzed by immunoblotting using anti-Drp1 and anti-p53. (C) Mitochondrial oxygen consumption of brain mitochondria from wild-type and R6/2 mice was analyzed after 8 weeks of TAT or P110-TAT treatment, and the RCR was calculated. *P < 0.05 vs. wild-type; #P < 0.01 vs. R6/2; n = 7/group). (D) P110-TAT treatment improved the mobility of R6/2 mice relative to TAT-treated R6/2 mice, to levels similar to wild-type mice treated with either peptide (R6/2 TAT, n = 9; R6/2 P110-TAT, n = 11; WT TAT, n = 12; WT P110-TAT, n = 12; *P < 0.05). (E and F) Rearing time (E) and frequency (F) were significantly lower in R6/2 mice treated with TAT, compared with R6/2 mice treated with P110-TAT and wild-type mice treated with either peptide (**P < 0.01). (G) Survival of R6/2 mice treated with TAT was significantly lower than for R6/2 mice treated with P110-TAT or wild-type mice (Mantel Cox log-rank test, χ2 = 17.27; 3 degrees of freedom; ***P = 0.0006; n = 12 per group). Data represent mean ± SEM.