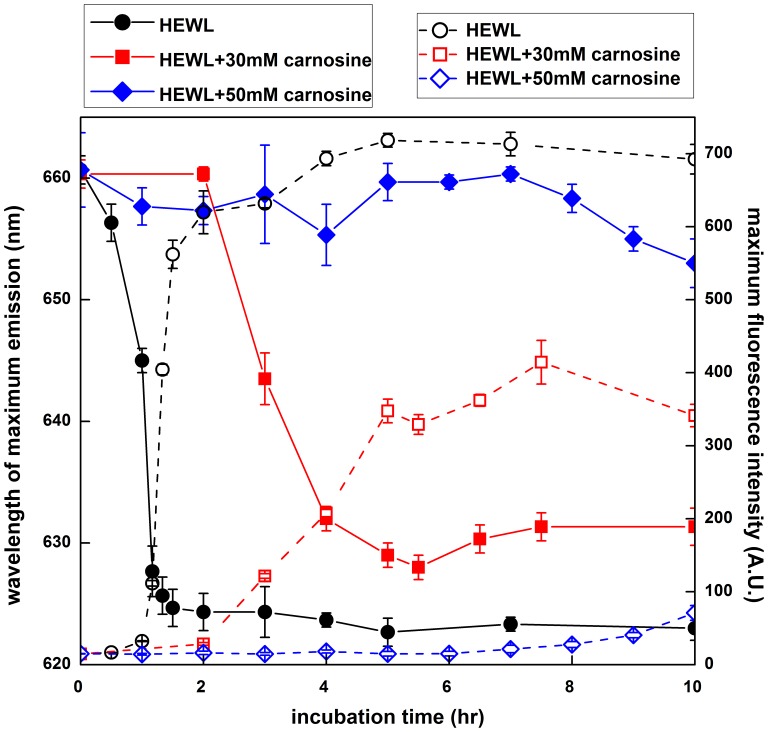
Figure 5. The effects of carnosine on the hydrophobicity of HEWL during incubation.
The time-course of the protein surface hydrophobicity was measured by Nile red-binding fluorescence at different incubation times. Data were presented as maximum Nile red fluorescence intensity and wavelength of maximum fluorescence emission taken at various incubation time. Each point represents the average of at least 5 independent measurements (n≥5). HEWL samples were dissolved in 100 mM glycine buffer (pH 2.0) and incubated at 55°C accompanied by agitation of 580 rpm during the course of the experiment (solid symbols represent the wavelength of maximum fluorescence and open symbols are indicative of maximum Nile red fluorescence intensity).