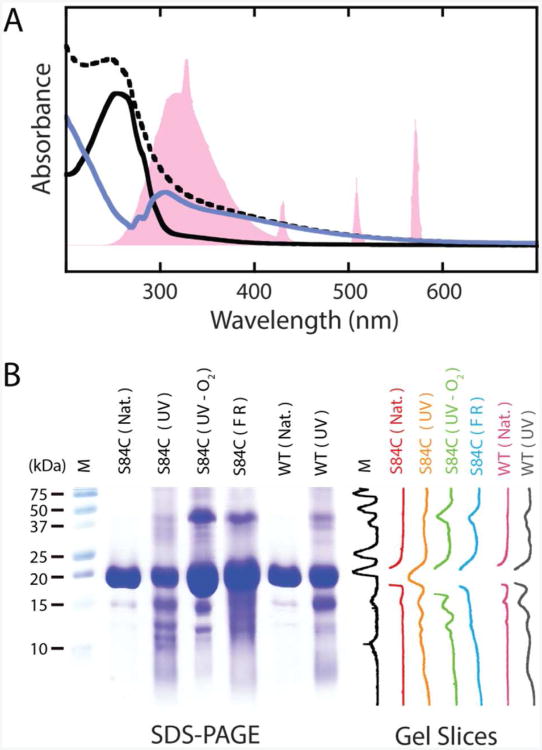
Figure 1.
UV-B photodamage products of human γD-crystallin. A. UV-vis spectra of γD-crystallin before and after UV-B illumination. The output spectrum of the mercury vapor lamp, normalized to the protein absorption, is shown in pink. The spectrum of the protein before illumination (solid, black) shows absorption below 300 nm consistent with the tyrosine and tryptophan content of the protein. After exposure to UV-B light, the spectrum (dashed, black) becomes broadened into the visible range. Subtraction of the pre-UV-B spectrum from the spectrum of the photodamaged protein yields a difference spectrum (solid, blue) with a bleach between 250 and 305 nm. The band structure of this bleach suggests a loss of tryptophan absorption. B. SDS-PAGE characterization of γD-crystallin degradation via UV-B photodamage and chemical methods. From left to right are molecular weight marker (M), undamaged γD-crystallin (S84C) (S84C (Nat.)), UV-B photodamaged γD-crystallin (S84C) (S84C (UV)), UV-B photodamaged γD-crystallin (S84C) in deoxygenated buffer (S84C (UV-O2)), γD-crystallin (S84C) exposed to Fenton's reagent (S84C (FR)), undamaged wild type γD-crystallin (WT (Nat.)), and UV-B photodamaged wild type γD-crystallin (WT (UV)), respectively. Vertical slices of the gel image are shown for comparison of each sample.