Abstract
The need for lengthy treatment to cure tuberculosis stems from phenotypic drug resistance, also known as drug tolerance, which has been previously attributed to slowed bacterial growth in vivo. We discuss recent findings that challenge this model and instead implicate macrophage-induced mycobacterial efflux pumps in antimicrobial tolerance. Although mycobacterial efflux pumps may have originally served to protect against environmental toxins, in the pathogenic mycobacteria they appear to have been repurposed for intracellular growth. In this light, we discuss the potential of efflux pump inhibitors such as verapamil to shorten tuberculosis treatment by their dual inhibition of tolerance and growth.
Drug tolerance is an important barrier to shortening TB treatment
The long duration of treatment required with current anti-tuberculous drugs presents a major challenge in tuberculosis (TB) management. At least six months of treatment are required to achieve acceptable cure and relapse rates for smear-positive tuberculosis (Connolly et al. 2007; Mitchison and Davies 2012). Although an important breakthrough when first introduced, such “short course” therapy is still too long. Adherence to months of TB therapy is difficult, with default rates of nearly 30% reported in some series (Castelnuovo 2010). The consequences of poor adherence are serious both for the individual patient and for the community: drug resistance, treatment failure, and further TB transmission. Attempts to shorten treatment to four months have been thwarted by unacceptably high relapse rates (Johnson et al. 2009).
Why is lengthy treatment with current medications required to cure TB? The answer may be found in observations from landmark TB studies. For years, it has been recognized that when patients with drug-susceptible TB relapse, the bacilli typically remain genetically drug-susceptible and patients respond to their prior treatment regimens (British Medical Research Council 1972; Wallis et al. 1999). Complementary data from early bactericidal activity studies by Jindani and Mitchison demonstrated that during TB chemotherapy, sputum bacillary counts decrease in a characteristic biphasic manner (Jindani et al. 1980). For example with isoniazid, greater than 99% of the initial sputum bacillary load is killed during the first two days of treatment, after which the rate of killing drops off markedly. The residual bacteria are a phenotypically resistant, “drug tolerant” population; TB drug minimum inhibitory concentrations are unchanged. Empirical studies have shown that it takes months of therapy to eradicate these bacteria and produce a stable cure (Mitchison and Davies 2012).
The phenomenon of antimicrobial tolerance was recognized in early experiments studying in vitro killing of streptococci and staphylococci by penicillin (Bigger 1944; Hobby et al. 1942) and was subsequently found to generalize to other bacteria, including Mycobacterium tuberculosis (Mtb) (McCune and Tompsett 1956; Wallis et al. 1999). Existing models of antimicrobial tolerance differ in their specifics but all invoke the presence of a metabolically quiescent, non-growing population. Older views focused on deterministic mechanisms such as hypoxia or nutrient starvation, conditions that are thought to occur in the tuberculous granuloma; more recent models implicate stochastic mechanisms whereby so-called “persister” cells arise independent of the growth environment (Dhar and McKinney 2007; Lewis 2010). Although tolerance models that emphasize a role for slow-growing or non-growing bacteria are compatible with the observation that antimicrobials kill non-growing TB poorly (Schaefer 1954), evidence from human treatment studies suggest that the drug-tolerant population may not in fact be quiescent. Serial radiological studies have demonstrated that existing lesions may enlarge and new lesions may develop despite an overall efficacious course of therapy, a phenomenon that may be explained by the presence of an enlarging, drug-tolerant Mtb population (Akira et al. 2000; Bobrowitz 1980).
Actively-growing intracellular mycobacteria exhibit multidrug tolerance mediated by macrophage-induced bacterial efflux pumps
Recent insights from the zebrafish-M. marinum (Mm) model of TB offer potential explanations for these puzzling radiographic observations. Similar to the expansion of a subset of tuberculous lesions during human therapy, drug-tolerant Mm continue to expand and disseminate within macrophages during infection of zebrafish (Adams et al. 2011). Further investigation with macrophage-like cell lines revealed that subpopulations of both Mtb and Mm become tolerant to multiple classes of antimicrobials including isoniazid and rifampicin upon intracellular residence. The induction of drug tolerance in bacteria by the host macrophage environment has been previously described for Legionella pneumophila (Barker et al. 1995) and may be a more widespread phenomenon. Countering prior models, the mycobacterial work revealed that macrophage-induced tolerance is enriched in actively-dividing bacteria (Figure 1) (Adams et al. 2011). This surprising result was explained by the finding that in Mtb, macrophage-induced tolerance to rifampicin is mediated by a bacterial efflux pump, Rv1258c, that also promotes intracellular bacterial growth in the absence of antimicrobials (Table 1) (Adams et al. 2011). Rv1258c, a secondary transporter belonging to the Major Facilitator Superfamily (MFS) of efflux pumps, is structurally related to MefA, a 12-membrane spanning MFS pump involved in macrolide resistance in Streptococcus pneumoniae (Ainsa et al. 1998; De Rossi et al. 2002; Li and Nikaido 2009; Saier et al. 2009). Rv1258c is transcriptionally induced following macrophage residence (Table 1) (Schnappinger et al. 2003) and appears to function as a virulence factor induced in the intracellular environment that pathogenic mycobacteria encounter (Chan et al. 2002; Clay et al. 2007; Dannenberg 1993; Ramakrishnan et al. 2000). Its association with rifampicin tolerance appears to be an epiphenomenon, and the identity of the “natural” substrate(s) of Rv1258c remains unknown. Indeed, despite decades of study there are still only a few clearly identified natural substrates of bacterial efflux pumps, such as spermidine in B. subtilis, bile salts in E. coli, and cyclic-di-AMP in Listeria monocytogenes (Thanassi et al. 1997; Woodward et al. 2010; Woolridge et al. 1997).
Figure 1. Model for Efflux Pump Inhibitor Action in Mycobacterium tuberculosis (Mtb).
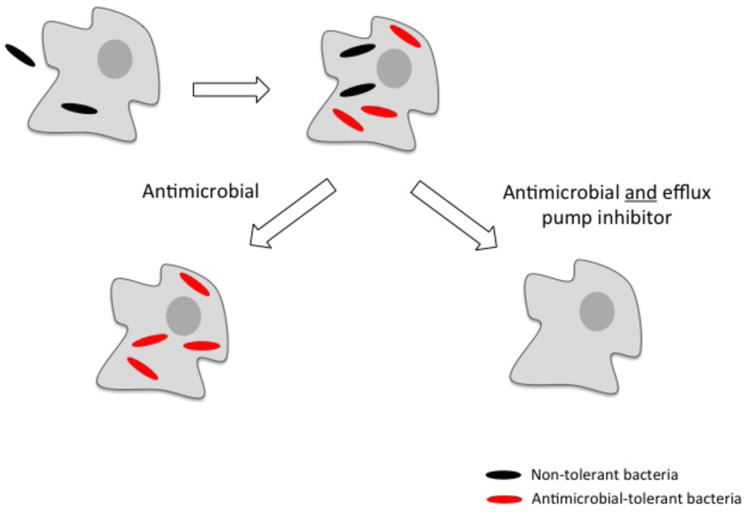
Efflux pump expression is induced in Mtb following macrophage residence, perhaps stimulated by macrophage antimicrobial peptides. With antimicrobial treatment alone, non-tolerant bacteria are killed, but tolerant bacteria survive and multiply within the macrophage. When antimicrobials are given in conjunction with an efflux pump inhibitor, the tolerant bacteria are killed along with non-tolerant bacteria. Note that in this simplified diagram there is no attempt to differentiate between mycobacterial residence in the cytoplasm versus the phagosome. Modified from (Adams et al. 2011)
Table 1. Macrophage-induced Mycobacterium tuberculosis efflux pumps.
| Drug Efflux Pump§ | Transporter Family | Macrophage growth attenuation* | Associated drug resistance | Homologs in other mycobacteria | References |
|---|---|---|---|---|---|
| Rv0194 | ABC | Yes | STR | M.marinum; M. ulcerans | (Braibant et al. 2000; Danilchanka et al. 2008) |
| Rv1218c | ABC | Yes | M. smegmatis; M. marinum; M. avium; M. leprae; M. abscessus | (Balganesh et al. 2012; Balganesh et al. 2010; Braibant et al. 2000) | |
| Rv1272c | ABC | Yes | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. leprae; M. abscessus | (Braibant et al. 2000) | |
| Rv1273c | ABC | Yes | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. leprae; M. abscessus | (Braibant et al. 2000) | |
| Rv1348 | ABC | ND | M. smegmatis; M.marinum; M. ulcerans; M. avium; M. abscessus | (Braibant et al. 2000; Farhana et al. 2008) | |
| Rv1349 | ABC | ND | M. smegmatis; M.marinum; M. ulcerans; M. avium; M. abscessus | (Braibant et al. 2000; Farhana et al. 2008) | |
| Rv1463 | ABC | ND | M. smegmatis; M.marinum; M. ulcerans; M. avium; M. abscessus | (Braibant et al. 2000) | |
| Rv1687c | ABC | No | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. leprae | (Braibant et al. 2000) | |
| Rv2686c | ABC | Yes | CIP | M. smegmatis; M.marinum; M. ulcerans, M. leprae; M. abscessus | (Braibant et al. 2000; Louw et al. 2009;Pasca et al. 2004) |
| Rv2687c | ABC | ND | CIP | M. smegmatis; M.marinum; M. ulcerans; M. abscessus | (Braibant et al. 2000; Pasca et al. 2004) |
| Rv2688c | ABC | No | CIP | M. smegmatis; M.marinum; M. ulcerans; M. abscessus | (Braibant et al. 2000; Pasca et al. 2004) |
| Rv1258c | MFS | Yes | RIF, OFX, INH | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. leprae; M. abscessus | (Balganesh et al. 2012; De Rossi et al. 2002; Jiang et al. 2008; Rodrigues et al. 2011b; Siddiqi et al. 2004; Zhang et al. 2005) |
| Rv3239c | MFS | No | M.marinum; M. ulcerans | (De Rossi et al. 2002; Louw et al. 2009) | |
| Rv3728 | MFS | No | M.marinum; M. ulcerans; M. leprae | (De Rossi et al. 2002; Gupta et al. 2010; Louw et al. 2009) | |
| Rv1183 (mmpL1 0) | RND | ND | M. smegmatis; M.marinum, M. avium; M. leprae; M. abscessus | (Tekaia et al. 1999) | |
| Rv1146 (mmpL13b) | RND | ND | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. abscessus | (Tekaia et al. 1999) | |
| Rv3065 (mmr) | SMR | ND | ERM | M.marinum; M. ulcerans, M. avium; M. leprae | (Balganesh et al. 2012; Gupta et al. 2010) |
| Rv0969 (ctpV) | Putative copper exporter | No | M. smegmatis, M. marinum, M, ulcerans, M. avium; M. abscessus | (Ward et al. 2010) | |
| Rv3578 (arsB2) | Probable arsenic pump | Yes | M. smegmatis; M.marinum; M. ulcerans, M. avium; M. leprae | (Ordonez et al. 2005) |
Isoniazid (INH); rifampicin (RIF); ofloxacin (OFX); ciprofloxacin (CIP); streptomycin (STR); ethambutol (EMB); erythromycin (ERM); ATP-binding cassette (ABC); major facilitator superfamily (MFS); resistance-nodulation cell division family (RND); small multidrug resistance family (SMR); not determined (ND)
Efflux pumps that are significantly induced (1.3 to 2.6-fold) at 48 hours in naïve macrophages (Schnappinger et al. 2003). Those highlighted in bold have been tested experimentally for efflux activity, all others are predicted efflux pumps based on homology to efflux pumps in other organisms.
Macrophage growth attenuation from resting or IFNγ-activated macrophages (Rengarajan et al. 2005)
The findings coupling intracellular bacterial growth and antimicrobial tolerance through induction of bacterial efflux pumps are compatible with the longstanding clinical observation that the duration of curative TB treatment is proportional to the organism burden. Indeed, the highest mycobacterial burden states, smear-positive and cavitary disease, require the longest therapy for a durable cure (Connolly et al. 2007; British Medical Research Council 1989; Zierski et al. 1980). Models of non-replicating tolerance have attributed this association to high burden disease having increased numbers of non-replicating as well as replicating bacteria (Connolly et al. 2007). However, the link between mycobacterial burden and treatment duration is equally well explained by the alternative model attributing tolerance to actively-growing bacteria. Indeed, this view implicates increased efflux activity as the driver of both high mycobacterial burden disease and antimicrobial tolerance; high burden disease states should therefore be enriched in tolerant bacteria.
The role of efflux pumps in promoting drug tolerance opens up a potentially powerful approach for shortening TB treatment. The use of efflux pump inhibitors would target not only bacterial growth, but also drug tolerance. In the laboratory setting, macrophage-induced tolerance is inhibited by verapamil, a calcium channel antagonist in clinical use for years, which has been shown to also inhibit multiple bacterial efflux pumps in vitro (Adams et al. 2011; Marquez 2005; Rodrigues et al. 2011a). Consistent with the observation that macrophage-induced pumps mediate intracellular survival, verapamil also reduces intracellular mycobacterial growth in the absence of antibiotics (Adams et al. 2011; Martins et al. 2008). This review will discuss these findings in the context of a current understanding of antimicrobial efflux in mycobacteria and other bacteria, with an emphasis on teleological, functional, and therapeutic considerations.
Macrophage-induced Mtb efflux pumps are virulence determinants
Although information about their natural substrates is lacking, extensive in vitro studies have shown that efflux pumps in both prokaryotic and eukaryotic organisms can extrude a variety of toxic agents such as antimicrobials and chemotherapeutic agents (Ho and Kim 2005; Li and Nikaido 2009). Much effort has focused on the role of efflux in antimicrobial resistance (Li and Nikaido 2009). For example, the so-called multi-drug resistance (MDR) pumps have been implicated in resistance to structurally diverse antimicrobial agents and contribute to the burden of bacterial drug resistance. Efflux-mediated antimicrobial resistance was initially reported in Escherichia coli (Ball et al. 1980; McMurry et al. 1980), but has subsequently been recognized in a wide range of organisms, including the often recalcitrant Pseudomonas aeruginosa and Acinetobacter baumanii (Coyne et al. 2011; Li et al. 1995). With the identification of the LfrA pump in M. smegmatis as a mediator of fluoroquinolone resistance (Liu et al. 1996), there has been a growing interest in the contributions of drug efflux in mycobacteria (da Silva et al. 2011; Louw et al. 2009). Efflux has also been proposed to account for isoniazid-induced tolerance in Mtb and may be mediated by the isoniazid-induced protein IniA (Colangeli et al. 2005; Viveiros et al. 2002).
Our identification of Rv1258c as a mediator of intracellular growth led us to investigate if mycobacterial efflux pumps are widely used for this critical virulence trait. Mining the published literature reveals that 19 of the 55 annotated efflux pumps in the Mtb genome are transcriptionally-induced in macrophages (Table 1) (Camus et al. 2002; Cole et al. 1998; Schnappinger et al. 2003). Of the 12 tested by mutational analysis, seven are required for intracellular growth (Rengarajan et al. 2005). Thus several macrophage-induced efflux pumps serve non-redundant roles in promoting intracellular growth. Moreover, Mtb efflux pumps not found induced in the 48-hour macrophage infection assay have virulence phenotypes at later points, in a seven-day macrophage infection assay and/or in mouse infection models (Bigi et al. 2004; Curry et al. 2005; Rengarajan et al. 2005; Sassetti and Rubin 2003; Schnappinger et al. 2003). These may represent virulence genes that are induced later in the course of macrophage residence or by specific environments in vivo such as the tuberculous granuloma (Chan et al. 2002; Ramakrishnan et al. 2000). Efflux pumps in Gram-negative bacteria have been linked to multiple virulence functions including gut colonization, and adherence and invasion of cultured cells (Table 2) (Piddock 2006b). It is likely that Mtb pumps participate in similar activities. That Mtb allocates a great number of efflux pumps to ensure intracellular survival is consistent with it being a central strategy for mycobacterial virulence (Cosma et al. 2003; Shepard 1957).
Table 2. Bacterial efflux pumps associated with virulence.
| Pump Family | Organism | Virulence phenotype | Proposed mechanism | Mediates antibiotic resistance | References |
|---|---|---|---|---|---|
| ABC | |||||
| DrrABC | Mtb | In vivo survival | Localization of phthiocerol dimycocerosate in cell wall | Yes | (Camacho et al. 2001; Choudhuri et al. 2002; Sassetti and Rubin 2003) |
| MacAB | Salmonella enterica serovar Typhimurium | In vivo survival | May detoxify host-derived molecules | Yes | (Nishino et al. 2006) |
| Rv1272c | Mtb | In vivo survival | Unknown | ND | (Sassetti and Rubin 2003) |
| Rv1747 | Mtb | Intracellular growth; in vivo survival | Substrate for PknF a serine threonine kinase involved in regulating glucose intake | ND | (Molle et al. 2004; Sassetti and Rubin 2003; Spivey et al. 2011) |
| Rv3781 | Mtb | In vivo survival | Maybe involved in arabinogalactan biosynthesis | ND | (Dianiskova et al. 2011; Sassetti and Rubin 2003) |
| MFS | |||||
| MdrM | Listeria monocytogenes | In vivo growth | Secretion of c-di-AMP | Yes | (Crimmins et al. 2008; Woodward et al. 2010) |
| MdrT | Listeria monocytogenes | In vivo growth | Secretion of c-di-AMP Cholic acid transporter | Yes | (Crimmins et al. 2008; Quillin et al. 2011; Woodward et al. 2010) |
| NorA | Staphylococcus aureus | Host cell invasion | Unknown | Yes | (Aeschlimann et al. 1999; DeMarco et al. 2007; Kalia et al. 2012) |
| NorB | Staphylococcus aureus | In vivo survival | Unknown | Yes | (DeMarco et al. 2007; Ding et al. 2008) |
| P55 (Rv1410c) | Mtb, Mycobacterium bovis | Intracellular growth; in vivo survival | Preservation of cell wall | Yes | (Bianco et al. 2011; Ramon-Garcia et al. 2009; Rengarajan et al. 2005; Sassetti and Rubin 2003) |
| QacA | Staphylococcus aureus | In vivo persistence | Increased membrane fluidity | Yes | (Bayer et al. 2006; Dhawan et al. 1997; Kupferwasser et al. 1999) |
| Rv0037c | Mtb | Intracellular growth | Unknown | ND | (Rengarajan et al. 2005) |
| Rv0849 | Mtb | Intracellular growth | Unknown | ND | (Rengarajan et al. 2005) |
| Tap (Rv1258c) | Mtb | Intracellular growth | Unknown | Yes | (Adams et al. 2011; Ainsa et al. 1998; Balganesh et al. 2012; Sharma et al. 2010; Siddiqi et al. 2004) |
| RND | |||||
| AcrAB | Escherichia coli, Francisella tularensis, Klebsiella pneumoniae, Salmonella enter ica serovar Typhimurium, Enterobacter cloacae | In vivo survival | Efflux of bile acids | Yes | (Bina et al. 2008a; Blair and Piddock 2009; Buckley et al. 2006; Helling et al. 2002; Ma et al. 1995; Padilla et al. 2010; Perez et al. 2012; Rosenberg et al. 2003; Thanassi et al. 1997) |
| BesC | Borrelia burgdorferi | In vivo survival | Possible component of type I secretion system | Yes | (Bunikis et al. 2008) |
| BpeAB-OprB | Burkholderia pseudomallei | Host cell invasion | Quorum sensing | Yes | (Chan and Chua 2005) |
| CmeABC | Campylobacter jejuni | In vivo colonization | Efflux of bile acids | Yes | (Lin and Martinez 2006; Lin et al. 2003; Martinez and Lin 2006) |
| MexCD-OprJ | Pseudomonas aeruginosa | In vivo survival; hyperexpressio n compromises expression of type III secretion genes | Secretion of quorum sensing molecules | Yes | (Join-Lambert et al. 2001; Linares et al. 2005) |
| MexEF-OprN | Pseudomonas aeruginosa | In vivo survival; hyperexpressio n compromises expression of type III secretion genes | Secretion of quorum sensing molecules | Yes | (Frisk et al. 2004; Join-Lambert et al. 2001; Kohler et al. 2001; Lamarche and Deziel 2011; Linares et al. 2005) |
| MmpL7 | Mtb | Intracellular growth; in vivo survival | Translocation of phthiocerol dimycocerosate to cell wall | Yes | (Camacho et al. 2001; Domenech et al. 2005; Lamichhane et al. 2005; Pasca et al. 2005; Rodrigues et al. 2011b; Sassetti and Rubin 2003) |
| MmpL10 | Mtb | In vivo survival | Unknown | ND | (Lamichhane et al. 2005; Sassetti and Rubin 2003) |
| MtrCDE | Neisseria gonorrhoeae, Neisseria meningitidis | In vivo survival | Resistance to host antimicrobial defenses | Yes | (Hagman et al. 1995; Jerse et al. 2003; Shafer et al. 1995; Shafer et al. 1998; Tzeng et al. 2005; Warner et al. 2008) |
| VexH | Vibrio cholerae | Intestinal colonization | Export of cholera toxin and toxin co-regulated pilus | Yes | (Bina et al. 2008b; Taylor et al. 2012) |
| TolC | Brucella suis, Francisella tularensis, Legionella pneumophila, Salmonella enterica serovar Typhimurium, Salmonella enter itidis | Intracellular growth; intestinal colonization | Might be involved in efflux of reactive oxygen species | Yes | (Buckley et al. 2006; Ferhat et al. 2009; Nishino et al. 2006; Platz et al. 2010; Posadas et al. 2007; Stone and Miller 1995; Wu et al. 2012) |
| Other | |||||
| ArsB2 | Mtb | Intracellular growth | Probable arsenic pump | ND | (Rengarajan et al. 2005) |
| CopA | Neisseria gonorrhoeae | Invasion and survival | Export of copper ions | ND | (Djoko et al. 2012) |
| CtpV | Mtb | Intracellular growth; in vivo survival | Putative copper exporter | ND | (Rengarajan et al. 2005; Ward et al. 2010) |
Mycobacterium tuberculosis (Mtb)
Mtb macrophage-induced efflux pumps: signals and substrates
The presence of distinct host-generated defenses within the macrophage may explain the observation that multiple macrophage-induced Mtb pumps are individually essential for intracellular growth. What are the stimuli that induce pump expression and what are the substrates? Do different pumps have shared stimuli but unique substrates? Although these answers are not clear, indirect clues suggest that tolerance-producing mycobacterial efflux pumps may be induced by antimicrobial peptides (AMPs) (Adams et al. 2011). Indeed, macrophage-induced M. marinum tolerance is not inhibited by dexamethasone, a glucocorticoid that reduces most macrophage defenses while sparing antimicrobial peptide expression (Adams et al. 2011; Duits et al. 2001; Ehrchen et al. 2007). This model has precedence: the macrophage-derived AMP LL-37 induces transcription mefE, which encodes one component of a Streptococcus pneumoniae efflux pump related to Rv1258c (Zahner et al. 2010).
Could AMPs also be substrates of the macrophage-induced efflux pumps? Studies predominantly from Gram-negative bacteria suggest this could be the case (Bengoechea and Skurnik 2000; Brissette and Lukehart 2007; Padilla et al. 2010; Shafer et al. 1998; Tzeng et al. 2005; Warner and Levy 2010). For example, in Neisseria gonorrhoeae, mutation of the Mtr efflux pump and treatment with the chemical efflux pump inhibitor carbonyl cynanide-m-chlorophenylhydrazone (CCCP) both increase AMP sensitivity as well as intracellular AMP accumulation (Shafer et al. 1998). Similar to the Rv1258c pump, Mtr also confers antibiotic resistance (Hagman et al. 1995). While Rv1258c mediates tolerance to the hydrophobic antibiotic rifampicin but not the hydrophilic isoniazid, Mtr similarly mediates resistance to rifampicin and the hydrophobic erythromycin, but not the hydrophilic antibiotic streptomycin. Though many pumps have been noted to have broad substrate promiscuity (Neyfakh 2002), these examples suggest that hydrophobic compounds may be transported by a more limited subset of pumps.
Of course, other mechanisms likely contribute to AMP resistance aside from AMP efflux (Kraus and Peschel 2006). While a comprehensive screen of Neisseria meningitidis mutants with increased susceptibility to an AMP-like cyclic lipopeptide revealed a predominance of mutations in the mtr gene, mutations in other genes involved in lipid A and pilin synthesis were also identified (Tzeng et al. 2005). In addition, S. aureus strains overexpressing the QacA pump showed evidence of decreased membrane fluidity (Bayer et al. 2006). Thus it would appear that resistance to AMPs can be mediated directly through efflux pumps as well as by compensatory mechanisms such as cell surface remodeling. In this context it is interesting that MmpL7, a Mtb efflux pump required for intracellular survival, is thought to exert its virulence effects by transporting phthiocerol dimycocerosates (PDIM) into the bacterial cell wall (Camacho et al. 2001; Cox et al. 1999). Similarly, a role in compensatory cell wall remodeling rather than direct drug transport may explain IniA's contribution to tolerance to isoniazid and ethambutol, drugs that act on the mycobacterial cell wall (Colangeli et al. 2005).
A consideration of the signals and substrates of the macrophage-induced Mtb pumps must account for two observations. First, only a subpopulation of intracellular bacteria exhibits antibiotic tolerance. The most likely explanation for this finding is that there is variation in efflux pump expression, with higher-expressing organisms attaining a drug-tolerant, macrophage growth-adapted phenotype. Why might pump expression vary? Variation could be stochastic or might occur if the pump-inducing signal is accessible to only a subset of bacteria, such as the subpopulation of Mm and Mtb that exit the phagosome into the cytostol (Stamm et al. 2003; van der Wel et al. 2007). However, Mm lacking RD1/ESX-1, which is required for cytosolic translocation (Simeone et al. 2012), still become drug-tolerant after macrophage residence (Adams et al. 2011); pump inducing signals must therefore be present in the phagosome. Of note, AMPs are known to access phagosomal bacteria; the macrophage-derived cathelicidin LL-37 has been shown to effectively kill Mtb in cell culture (Liu et al. 2006; Liu et al. 2007).
Second, in advanced human TB, most bacteria reside in the granuloma's necrotic core, known as the caseum (Canetti 1955; Grosset 2003). However, the effects of macrophage-induced tolerance may still be relevant after Mtb has exited macrophages. Down-regulation of efflux pumps may occur relatively slowly and may be balanced by an influx of “freshly tolerant” Mtb brought in by phagocytes that traffic into and lyse within the necrotic caseum (Cosma et al. 2004; Dannenberg 2003). Alternatively, the original stimulus may persist after macrophage lysis; in support of this hypothesis is the finding that macrophage-induced tolerance lasts for at least 5 days in vitro following macrophage lysis (Adams et al. 2011). Finally, additional stimuli in the extracellular environment could also maintain tolerance. Again, AMPs remain viable candidates as they can be produced by a diversity of cell types, including the respiratory epithelium (Parker and Prince 2011).
Function and regulation of macrophage-induced efflux: a teleological perspective
It is remarkable that the majority of the Mtb macrophage-induced efflux pumps, including those demonstrated to mediate intracellular growth, are widely conserved among mycobacteria with divergent lifestyles, ranging from the environmental Mycobacterium smegmatis to the ultimately host-adapted Mycobacterium leprae that is incapable of axenic growth (Tables 1 and 3) (Cole et al. 2001; Tsukamura 1976). Regulation of these pumps may also be conserved, as seen with Rv1258c. In Mtb its expression is under the transcriptional control of WhiB7, which belongs to an ancient and highly-conserved family of transcriptional regulators found in multiple actinomycetes including the soil-dwelling Streptomyces, Nocardia, and both environmental and pathogenic mycobacteria (Morris et al. 2005). WhiB7 mediates the characteristic low-level intrinsic resistance of Streptomyces and mycobacteria to antimicrobials of multiple classes (Morris et al. 2005). It is induced in response to sub-inhibitory concentrations of antimicrobials and mediates Rv1258c transcription in these settings. Furthermore, WhiB7 is itself induced by macrophage residence (Larsson et al. 2012; Rohde et al. 2012), and would also be predicted to be required for Rv1258c transcriptional induction and bacterial survival in this context.
Table 3. Mycobacterial Species with Homologs of Mycobacterium tuberculosis Macrophage-Induced Pumps.
| Species (Genome Size)§ | Environmental Niche | Natural Vertebrate Host | Host Niche | Associated Human Disease(s) | Treatment* | References |
|---|---|---|---|---|---|---|
| M. smegmatis(7MB) | Soil | None known | Not applicable | Extremely rare. Case reports primarily of localized disease, e.g. wound infections. | Optimal therapy unknown; resistant to multiple drugs including rifampicin. | (Long et al. 2012; Pierre-Audigier et al. 1997; Tsukamura 1976; Wallace et al. 1988) |
| M. marinum(6.6 MB) | Water ?Amoebae | Fish Amphibians | Intra and extracellular | “Fish tank granuloma” | clarithromycin OR minocycline OR rifampicin plus ethambutol | (Linell and Norden 1954; Stinear et al. 2008; Yanong et al. 2010) |
| M. ulcerans (5.6 MB) | ?Aquatic insects | None known | Mainly extracellular after brief intracellular phase | Buruli ulcer | Surgery rifampicin and streptomycin | (Doig et al. 2012; George et al. 1999; Wansbrough-Jones and Phillips 2006) |
| M. avium complex (5.5 MB) | Soil and water ?Amoebae Insects, earthworms | Birds, domesticated and non-domesticated mammals | Intracellular | Pulmonary and systemic infections, especially in the immunocompromised. | Pulmonary disease: clarithromycin OR azithromycin PLUS rifampicin and ethambutol, with or without an aminoglycoside | (Beumer et al. 2010; Biet et al. 2005; Falkinham 2010; Falkinham et al. 2001; Yamazaki et al. 2006) |
| M. abscessus (5.1 MB) | Water ?Amoebae | Fish Amphibians | Intra and extracellular | Pneumonia, soft tissue infection, disseminated infection in the immunocompromised | Multidrug resistant including to rifampicin Pulmonary and disseminated infection unlikely to be cured. Amikacin, imipenem, linezolid, tigecycline retain activity | (Medjahed et al. 2010; Nessar et al. 2012; Ripoll et al. 2009) |
| M. tuberculosis (4.4 MB) | None known | Humans | Intra and extracellular | Tuberculosis | isoniazid rifampicin pyrazinamide ethambutol | (Grosset 2003; Kumar and Rao 2011) |
| M. leprae (3.3 MB) | None known | Humans Recently introduced into armadillos | Intracellular | Leprosy | dapsone and rifampicin, with clofazamine added for multibacillary disease | (Rodrigues and Lockwood 2011; Singh and Cole 2011) |
Despite the varied environments different mycobacterial species face, they may share signals and substrates for efflux pumps. Environmental mycobacteria like M. smegmatis may be using pumps to defend against small molecules such as lantibiotics and antibiotics produced by environmental competitors and perhaps enhance growth within free-living amoebae (Asaduzzaman and Sonomoto 2009; Lamrabet et al. 2012). The capacity to extrude AMP-like peptides may have allowed mycobacteria to expand further into intracellular niches and thereby to a wide range of complex hosts. While the strictly host-adapted bacteria like Mtb and M. leprae have not been subjected to environmental antibiotic pressure for millennia, these skills again found use with introduction of antibiotics into medical practice in the 20th century.
Therapeutic implications for drug tolerance
The conservation of these macrophage-induced pumps in a range of pathogenic mycobacteria suggests their inhibition may constitute a therapeutic strategy not only for TB but for other difficult to treat mycobacterial diseases like leprosy, Buruli ulcer, and pulmonary infections with M. avium (Table 3). Indeed, Rv1258c has homologs in these species and rifampicin plays an important part in their treatment (Tables 1 and 3). Multiple drugs - verapamil, reserpine, phenothiazines such as thioridazine, and piperine - have been shown to inhibit bacterial efflux pumps in vitro (Kaatz 2005; Marquez 2005; Rodrigues et al. 2011a; Sharma et al. 2010). In general, the mechanisms by which these agents act are poorly understood. Several models have been proposed, such as: 1) direct binding and inhibition of pump assembly or function; 2) disruption of the transmembrane gradients utilized by secondary transporters; 3) inhibitor binding to the antimicrobial compound; 4) competition for efflux (Marquez 2005; Martins et al. 2008; Pages and Amaral 2009; Piddock 2006a). It is worth noting that some of these efflux pump inhibitors may also block macrophage antibiotic efflux, leading to increased intracellular drug levels (Cao et al. 1992), an effect that would potentiate their effect on the bacteria.
Verapamil, a calcium channel antagonist long in clinical use, is perhaps the most promising inhibitor for further evaluation as an adjunctive TB agent given its ability to reverse macrophage-induced tolerance to rifampicin (Adams et al. 2011). Other candidates include piperine, a derivative of black pepper that has been proposed to inhibit Mtb efflux pumps including Rv1258c (Sharma et al. 2010; Srinivasan 2007); agents developed to counter Gram-negative efflux pumps such as the Phe-Arg-β-naphthylamine derivatives (Pages and Amaral 2009); and P-glycoprotein inhibitors originally studied in cancer such as tariquidar (Leitner et al. 2011). The greatest benefit may come from approaches that inhibit multiple pumps, either through broadly-acting inhibitors or a combination of more specific inhibitors.
Could clinically significant resistance to efflux pump inhibitors arise? Certainly these compounds could be vulnerable to many of the same mycobacterial defensive measures used against traditional antimicrobials such as decreased membrane permeability, chemical inactivation of the inhibitor, pump overexpression, pump mutation (Ahmed et al. 1993; Klyachko et al. 1997), or efflux of the inhibitor (Garvey and Piddock 2008). It is possible though that the barrier to resistance for efflux pump inhibitors is higher than with traditional antimicrobials. For example, alteration of a binding site on one pump might not be sufficient to confer inhibitor resistance, if the inhibitor can target multiple bacterial pumps involved in tolerance. Moreover, inhibitors that additionally act on macrophage efflux may present a further barrier to evolution of resistance.
Efflux pump inhibition for drug-resistant TB
Appreciation for the potential of efflux pump inhibition strategies in drug-tolerant Mtb is recent, but joins a growing interest in developing this strategy for genetically drug-resistant Mtb (Amaral et al. 2010), where current treatment options are limited by even longer duration and increased toxicity (World Health Organization 2011). A substantial proportion of drug-resistant isolates have no identifiable mutations in known resistance-associated genes, and it appears that resistance in some of these isolates may result from increased efflux activity (Louw et al. 2009). In fact, multiple studies have reported increased efflux pump expression in Mtb clinical isolates (Gupta et al. 2006; Gupta et al. 2010; Hao et al. 2011; Jiang et al. 2008; Siddiqi et al. 2004). Accordingly, efflux pump inhibitors have been shown to reduce isoniazid, ciprofloxacin, ofloxacin, streptomycin, and linezolid minimum inhibitory concentrations in resistant strains (Escribano et al. 2007; Machado et al. 2012; Richter et al. 2007; Rodrigues et al. 2012; Singh et al. 2011; Spies et al. 2008). Although in vivo data are limited, a promising recent study found that verapamil restored activity of isoniazid, rifampicin, and pyrazinamide against MDR-TB in mice (Louw et al. 2011).
Efflux has been generally associated with low level intrinsic drug resistance, which may nevertheless exceed clinical breakpoints and can be further amplified by pumps of overlapping substrate specificities (Lee et al. 2000; Piddock 2006b). Moreover, this resistance may confer a survival advantage during antibiotic treatment that allows other chromosomal mutations to accumulate, further increasing the degree of antimicrobial resistance (Srivastava et al. 2010). Efflux pump activity also appears able to induce cross resistance to structurally and mechanistically diverse compounds: rifampicin treatment of rifampicin-resistant Mtb induced resistance to ofloxacin, which could be reversed with efflux pump inhibitors (Louw et al. 2011). The clinical implications of this phenomenon are quite serious. In areas where access to mycobacterial cultures are limited, the standard TB regiments prescribed to patients with unrecognized drug-resistant TB may not only have minimal efficacy, they may serve to further limit treatment options. Thus, the addition of efflux pump inhibitors to overcome drug-tolerance may have the additional benefit of reducing emergence of genetically drug-resistant Mtb.
Conclusions
Long recognized as a common mechanism of genetic antimicrobial resistance, efflux pump activity may play a dual role in Mtb, contributing to both virulence and drug tolerance. These pumps may have originally served to defend against environmental toxins that included antibiotics, but came to be utilized by pathogenic mycobacteria for intracellular survival. It is intriguing that their ancient function has come “full circle” - these pumps provide Mtb with a survival advantage in the era of antituberculous chemotherapy. The demonstration of verapamil's activity against macrophage-induced tolerance in the laboratory warrants its assessment in TB patients to determine if it will permit treatment shortening. Further understanding of efflux mediated drug tolerance may pave the way for new efflux pump inhibitors as well as complementary strategies to kill drug-tolerant mycobacteria.
Supplementary Material
Acknowledgments
JDS is supported by NIH T32 5T32AI007044-36 and the Merle A. Sande - Pfizer Fellowship in International Infectious Diseases. KNA is supported by NIH T32 AI55396. PHE is supported by NIH 5R18HS020002-03. LR is supported by grants from the NIH and is the recipient of the NIH Director's Pioneer Award.
We thank Josh Woodward, Christine Cosma, and Mark Troll for helpful discussions and manuscript review.
References
- Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell. 2011;145:39–53. doi: 10.1016/j.cell.2011.02.022. 10.1016/j.cell.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aeschlimann JR, Kaatz GW, Rybak MJ. The effects of NorA inhibition on the activities of levofloxacin, ciprofloxacin and norfloxacin against two genetically related strains of Staphylococcus aureus in an in-vitro infection model. The Journal of antimicrobial chemotherapy. 1999;44:343–9. doi: 10.1093/jac/44.3.343. [DOI] [PubMed] [Google Scholar]
- Ahmed M, Borsch CM, Neyfakh AA, Schuldiner S. Mutants of the Bacillus subtilis multidrug transporter Bmr with altered sensitivity to the antihypertensive alkaloid reserpine. The Journal of biological chemistry. 1993;268:11086–9. [PubMed] [Google Scholar]
- Ainsa JA, Blokpoel MC, Otal I, Young DB, De Smet KA, Martin C. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. Journal of bacteriology. 1998;180:5836–43. doi: 10.1128/jb.180.22.5836-5843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira M, Sakatani M, Ishikawa H. Transient radiographic progression during initial treatment of pulmonary tuberculosis: CT findings. Journal of computer assisted tomography. 2000;24:426–31. doi: 10.1097/00004728-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soolingen D. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! International journal of antimicrobial agents. 2010;35:524–6. doi: 10.1016/j.ijantimicag.2009.12.019. 10.1016/j.ijantimicag.2009.12.019. [DOI] [PubMed] [Google Scholar]
- Asaduzzaman SM, Sonomoto K. Lantibiotics: diverse activities and unique modes of action. Journal of bioscience and bioengineering. 2009;107:475–87. doi: 10.1016/j.jbiosc.2009.01.003. 10.1016/j.jbiosc.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Balganesh M, Dinesh N, Sharma S, Kuruppath S, Nair AV, Sharma U. Efflux Pumps of Mycobacterium tuberculosis Play a Significant Role in Antituberculosis Activity of Potential Drug Candidates. Antimicrobial agents and chemotherapy. 2012;56:2643–51. doi: 10.1128/AAC.06003-11. 10.1128/AAC.06003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balganesh M, Kuruppath S, Marcel N, Sharma S, Nair A, Sharma U. Rv1218c, an ABC transporter of Mycobacterium tuberculosis with implications in drug discovery. Antimicrobial agents and chemotherapy. 2010;54:5167–72. doi: 10.1128/AAC.00610-10. 10.1128/AAC.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball PR, Shales SW, Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochemical and biophysical research communications. 1980;93:74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Barker J, Scaife H, Brown MR. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrobial agents and chemotherapy. 1995;39:2684–8. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AS, Kupferwasser LI, Brown MH, Skurray RA, Grkovic S, Jones T, Mukhopadhay K, Yeaman MR. Low-level resistance of Staphylococcus aureus to thrombin-induced platelet microbicidal protein 1 in vitro associated with qacA gene carriage is independent of multidrug efflux pump activity. Antimicrobial agents and chemotherapy. 2006;50:2448–54. doi: 10.1128/AAC.00028-06. 10.1128/AAC.00028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengoechea JA, Skurnik M. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Molecular microbiology. 2000;37:67–80. doi: 10.1046/j.1365-2958.2000.01956.x. [DOI] [PubMed] [Google Scholar]
- Beumer A, King D, Donohue M, Mistry J, Covert T, Pfaller S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Applied and environmental microbiology. 2010;76:7367–70. doi: 10.1128/AEM.00730-10. 10.1128/AEM.00730-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco MV, Blanco FC, Imperiale B, Forrellad MA, Rocha RV, Klepp LI, Cataldi AA, Morcillo N, Bigi F. Role of P27 -P55 operon from Mycobacterium tuberculosis in the resistance to toxic compounds. BMC infectious diseases. 2011;11:195. doi: 10.1186/1471-2334-11-195. 10.1186/1471-2334-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biet F, Boschiroli ML, Thorel MF, Guilloteau LA. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC) Veterinary research. 2005;36:411–36. doi: 10.1051/vetres:2005001. 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]
- Bigger J. Treatment of Staphylococcal infections with Penicillin. The Lancet. 1944:497–500. [Google Scholar]
- Bigi F, Gioffre A, Klepp L, Santangelo MP, Alito A, Caimi K, Meikle V, Zumarraga M, Taboga O, Romano MI, Cataldi A. The knockout of the lprG-Rv1410 operon produces strong attenuation of Mycobacterium tuberculosis. Microbes and infection / Institut Pasteur. 2004;6:182–7. doi: 10.1016/j.micinf.2003.10.010. 10.1016/j.micinf.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Bina XR, Lavine CL, Miller MA, Bina JE. The AcrAB RND efflux system from the live vaccine strain of Francisella tularensis is a multiple drug efflux system that is required for virulence in mice. FEMS microbiology letters. 2008a;279:226–33. doi: 10.1111/j.1574-6968.2007.01033.x. 10.1111/j.1574-6968.2007.01033.x. [DOI] [PubMed] [Google Scholar]
- Bina XR, Provenzano D, Nguyen N, Bina JE. Vibrio cholerae RND family efflux systems are required for antimicrobial resistance, optimal virulence factor production, and colonization of the infant mouse small intestine. Infection and immunity. 2008b;76:3595–605. doi: 10.1128/IAI.01620-07. 10.1128/IAI.01620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JM, Piddock LJ. Structure, function and inhibition of RND efflux pumps in Gram-negative bacteria: an update. Current opinion in microbiology. 2009;12:512–9. doi: 10.1016/j.mib.2009.07.003. 10.1016/j.mib.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Bobrowitz ID. Reversible roentgenographic progression in the initial treatment of pulmonary tuberculosis. The American review of respiratory disease. 1980;121:735–42. doi: 10.1164/arrd.1980.121.4.735. [DOI] [PubMed] [Google Scholar]
- Braibant M, Gilot P, Content J. The ATP binding cassette (ABC) transport systems of Mycobacterium tuberculosis. FEMS microbiology reviews. 2000;24:449–67. doi: 10.1111/j.1574-6976.2000.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Brissette CA, Lukehart SA. Mechanisms of decreased susceptibility to beta-defensins by Treponema denticola. Infection and immunity. 2007;75:2307–15. doi: 10.1128/IAI.01718-06. 10.1128/IAI.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley AM, Webber MA, Cooles S, Randall LP, La Ragione RM, Woodward MJ, Piddock LJ. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cellular microbiology. 2006;8:847–56. doi: 10.1111/j.1462-5822.2005.00671.x. 10.1111/j.1462-5822.2005.00671.x. [DOI] [PubMed] [Google Scholar]
- Bunikis I, Denker K, Ostberg Y, Andersen C, Benz R, Bergstrom S. An RND-type efflux system in Borrelia burgdorferi is involved in virulence and resistance to antimicrobial compounds. PLoS pathogens. 2008;4:e1000009. doi: 10.1371/journal.ppat.1000009. 10.1371/journal.ppat.1000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho LR, Constant P, Raynaud C, Laneelle MA, Triccas JA, Gicquel B, Daffe M, Guilhot C. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. The Journal of biological chemistry. 2001;276:19845–54. doi: 10.1074/jbc.M100662200. 10.1074/jbc.M100662200. [DOI] [PubMed] [Google Scholar]
- Camus JC, Pryor MJ, Medigue C, Cole ST. Re-annotation of the genome sequence of Mycobacterium tuberculosis H37Rv. Microbiology. 2002;148:2967–73. doi: 10.1099/00221287-148-10-2967. [DOI] [PubMed] [Google Scholar]
- Canetti G. Histobacteriology and Its Bearing on the Therapy of Pulmonary Tuberculosis. Springer; New York: 1955. The Tubercle Bacillus in the Pulmonary Lesion of Man. [Google Scholar]
- Cao CX, Silverstein SC, Neu HC, Steinberg TH. J774 macrophages secrete antibiotics via organic anion transporters. The Journal of infectious diseases. 1992;165:322–8. doi: 10.1093/infdis/165.2.322. [DOI] [PubMed] [Google Scholar]
- Castelnuovo B. A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in Sub Saharan Africa. African health sciences. 2010;10:320–4. [PMC free article] [PubMed] [Google Scholar]
- Chan K, Knaak T, Satkamp L, Humbert O, Falkow S, Ramakrishnan L. Complex pattern of Mycobacterium marinum gene expression during long-term granulomatous infection. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3920–5. doi: 10.1073/pnas.002024599. 10.1073/pnas.002024599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YY, Chua KL. The Burkholderia pseudomallei BpeAB-OprB efflux pump: expression and impact on quorum sensing and virulence. Journal of bacteriology. 2005;187:4707–19. doi: 10.1128/JB.187.14.4707-4719.2005. 10.1128/JB.187.14.4707-4719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauty A, Ardant MF, Adeye A, Euverte H, Guedenon A, Johnson C, Aubry J, Nuermberger E, Grosset J. Promising clinical efficacy of streptomycinrifampin combination for treatment of buruli ulcer (Mycobacterium ulcerans disease) Antimicrobial agents and chemotherapy. 2007;51:4029–35. doi: 10.1128/AAC.00175-07. 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhuri BS, Bhakta S, Barik R, Basu J, Kundu M, Chakrabarti P. Overexpression and functional characterization of an ABC (ATP-binding cassette) transporter encoded by the genes drrA and drrB of Mycobacterium tuberculosis. The Biochemical journal. 2002;367:279–85. doi: 10.1042/BJ20020615. 10.1042/BJ20020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay H, Davis JM, Beery D, Huttenlocher A, Lyons SE, Ramakrishnan L. Dichotomous role of the macrophage in early Mycobacterium marinum infection of the zebrafish. Cell host & microbe. 2007;2:29–39. doi: 10.1016/j.chom.2007.06.004. 10.1016/j.chom.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangeli R, Helb D, Sridharan S, Sun J, Varma-Basil M, Hazbon MH, Harbacheuski R, Megjugorac NJ, Jacobs WR, Jr, Holzenburg A, Sacchettini JC, Alland D. The Mycobacterium tuberculosis iniA gene is essential for activity of an efflux pump that confers drug tolerance to both isoniazid and ethambutol. Molecular microbiology. 2005;55:1829–40. doi: 10.1111/j.1365-2958.2005.04510.x. 10.1111/j.1365-2958.2005.04510.x. [DOI] [PubMed] [Google Scholar]
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Krogh A, McLean J, Moule S, Murphy L, Oliver K, Osborne J, Quail MA, Rajandream MA, Rogers J, Rutter S, Seeger K, Skelton J, Squares R, Squares S, Sulston JE, Taylor K, Whitehead S, Barrell BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–44. doi: 10.1038/31159. 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honore N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG. Massive gene decay in the leprosy bacillus. Nature. 2001;409:1007–11. doi: 10.1038/35059006. 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS medicine. 2007;4:e120. doi: 10.1371/journal.pmed.0040120. 10.1371/journal.pmed.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Controlled clinical trial of short-course (6-month) regimens of chemotherapy for treatment of pulmonary tuberculosis. Lancet. 1972;1:1079–85. [PubMed] [Google Scholar]
- A controlled trial of 3-month, 4-month, and 6-month regimens of chemotherapy for sputum-smear-negative pulmonary tuberculosis. Results at 5 years. Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council. The American review of respiratory disease. 1989;139:871–6. doi: 10.1164/ajrccm/139.4.871. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Humbert O, Ramakrishnan L. Superinfecting mycobacteria home to established tuberculous granulomas. Nature immunology. 2004;5:828–35. doi: 10.1038/ni1091. 10.1038/ni1091. [DOI] [PubMed] [Google Scholar]
- Cosma CL, Sherman DR, Ramakrishnan L. The secret lives of the pathogenic mycobacteria. Annual review of microbiology. 2003;57:641–76. doi: 10.1146/annurev.micro.57.030502.091033. 10.1146/annurev.micro.57.030502.091033. [DOI] [PubMed] [Google Scholar]
- Cox JS, Chen B, McNeil M, Jacobs WR., Jr Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature. 1999;402:79–83. doi: 10.1038/47042. 10.1038/47042. [DOI] [PubMed] [Google Scholar]
- Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrobial agents and chemotherapy. 2011;55:947–53. doi: 10.1128/AAC.01388-10. 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins GT, Herskovits AA, Rehder K, Sivick KE, Lauer P, Dubensky TW, Jr, Portnoy DA. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10191–6. doi: 10.1073/pnas.0804170105. 10.1073/pnas.0804170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JM, Whalan R, Hunt DM, Gohil K, Strom M, Rickman L, Colston MJ, Smerdon SJ, Buxton RS. An ABC transporter containing a forkhead-associated domain interacts with a serine-threonine protein kinase and is required for growth of Mycobacterium tuberculosis in mice. Infection and immunity. 2005;73:4471–7. doi: 10.1128/IAI.73.8.4471-4477.2005. 10.1128/IAI.73.8.4471-4477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva PE, Von Groll A, Martin A, Palomino JC. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS immunology and medical microbiology. 2011;63:1–9. doi: 10.1111/j.1574-695X.2011.00831.x. 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- Danilchanka O, Mailaender C, Niederweis M. Identification of a novel multidrug efflux pump of Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2008;52:2503–11. doi: 10.1128/AAC.00298-08. 10.1128/AAC.00298-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg AM., Jr Immunopathogenesis of pulmonary tuberculosis. Hospital practice. 1993;28:51–8. doi: 10.1080/21548331.1993.11442738. [DOI] [PubMed] [Google Scholar]
- Dannenberg AM., Jr Macrophage turnover, division and activation within developing, peak and “healed” tuberculous lesions produced in rabbits by BCG. Tuberculosis. 2003;83:251–60. doi: 10.1016/s1472-9792(03)00048-9. [DOI] [PubMed] [Google Scholar]
- De Rossi E, Arrigo P, Bellinzoni M, Silva PA, Martin C, Ainsa JA, Guglierame P, Riccardi G. The multidrug transporters belonging to major facilitator superfamily in Mycobacterium tuberculosis. Molecular medicine. 2002;8:714–24. [PMC free article] [PubMed] [Google Scholar]
- DeMarco CE, Cushing LA, Frempong-Manso E, Seo SM, Jaravaza TA, Kaatz GW. Efflux-related resistance to norfloxacin, dyes, and biocides in bloodstream isolates of Staphylococcus aureus. Antimicrobial agents and chemotherapy. 2007;51:3235–9. doi: 10.1128/AAC.00430-07. 10.1128/AAC.00430-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar N, McKinney JD. Microbial phenotypic heterogeneity and antibiotic tolerance. Current opinion in microbiology. 2007;10:30–8. doi: 10.1016/j.mib.2006.12.007. 10.1016/j.mib.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Dhawan VK, Yeaman MR, Cheung AL, Kim E, Sullam PM, Bayer AS. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infection and immunity. 1997;65:3293–9. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianiskova P, Kordulakova J, Skovierova H, Kaur D, Jackson M, Brennan PJ, Mikusova K. Investigation of ABC transporter from mycobacterial arabinogalactan biosynthetic cluster. General physiology and biophysics. 2011;30:239–50. doi: 10.4149/gpb_2011_03_239. 10.4149/gpb_2011_03_239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Onodera Y, Lee JC, Hooper DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. Journal of bacteriology. 2008;190:7123–9. doi: 10.1128/JB.00655-08. 10.1128/JB.00655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djoko KY, Franiek JA, Edwards JL, Falsetta ML, Kidd SP, Potter AJ, Chen NH, Apicella MA, Jennings MP, McEwan AG. Phenotypic characterization of a copA mutant of Neisseria gonorrhoeae identifies a link between copper and nitrosative stress. Infection and immunity. 2012;80:1065–71. doi: 10.1128/IAI.06163-11. 10.1128/IAI.06163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig KD, Holt KE, Fyfe JA, Lavender CJ, Eddyani M, Portaels F, Yeboah-Manu D, Pluschke G, Seemann T, Stinear TP. On the origin of Mycobacterium ulcerans, the causative agent of buruli ulcer. BMC genomics. 2012;13:258. doi: 10.1186/1471-2164-13-258. 10.1186/1471-2164-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech P, Reed MB, Barry CE., 3rd Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infection and immunity. 2005;73:3492–501. doi: 10.1128/IAI.73.6.3492-3501.2005. 10.1128/IAI.73.6.3492-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits LA, Rademaker M, Ravensbergen B, van Sterkenburg MA, van Strijen E, Hiemstra PS, Nibbering PH. Inhibition of hBD-3, but not hBD-1 and hBD-2, mRNA expression by corticosteroids. Biochemical and biophysical research communications. 2001;280:522–5. doi: 10.1006/bbrc.2000.4157. 10.1006/bbrc.2000.4157. [DOI] [PubMed] [Google Scholar]
- Ehrchen J, Steinmuller L, Barczyk K, Tenbrock K, Nacken W, Eisenacher M, Nordhues U, Sorg C, Sunderkotter C, Roth J. Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood. 2007;109:1265–74. doi: 10.1182/blood-2006-02-001115. 10.1182/blood-2006-02-001115. [DOI] [PubMed] [Google Scholar]
- Escribano I, Rodriguez JC, Llorca B, Garcia-Pachon E, Ruiz M, Royo G. Importance of the efflux pump systems in the resistance of Mycobacterium tuberculosis to fluoroquinolones and linezolid. Chemotherapy. 2007;53:397–401. doi: 10.1159/000109769. 10.1159/000109769. [DOI] [PubMed] [Google Scholar]
- Falkinham JO., 3rd Hospital water filters as a source of Mycobacterium avium complex. Journal of medical microbiology. 2010;59:1198–202. doi: 10.1099/jmm.0.022376-0. 10.1099/jmm.0.022376-0. [DOI] [PubMed] [Google Scholar]
- Falkinham JO, 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. 2001;67:1225–31. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhana A, Kumar S, Rathore SS, Ghosh PC, Ehtesham NZ, Tyagi AK, Hasnain SE. Mechanistic insights into a novel exporter-importer system of Mycobacterium tuberculosis unravel its role in trafficking of iron. PloS one. 2008;3:e2087. doi: 10.1371/journal.pone.0002087. 10.1371/journal.pone.0002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhat M, Atlan D, Vianney A, Lazzaroni JC, Doublet P, Gilbert C. The TolC protein of Legionella pneumophila plays a major role in multi-drug resistance and the early steps of host invasion. PloS one. 2009;4:e7732. doi: 10.1371/journal.pone.0007732. 10.1371/journal.pone.0007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisk A, Schurr JR, Wang G, Bertucci DC, Marrero L, Hwang SH, Hassett DJ, Schurr MJ. Transcriptome analysis of Pseudomonas aeruginosa after interaction with human airway epithelial cells. Infection and immunity. 2004;72:5433–8. doi: 10.1128/IAI.72.9.5433-5438.2004. 10.1128/IAI.72.9.5433-5438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey MI, Piddock LJ. The efflux pump inhibitor reserpine selects multidrug-resistant Streptococcus pneumoniae strains that overexpress the ABC transporters PatA and PatB. Antimicrobial agents and chemotherapy. 2008;52:1677–85. doi: 10.1128/AAC.01644-07. 10.1128/AAC.01644-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George KM, Chatterjee D, Gunawardana G, Welty D, Hayman J, Lee R, Small PL. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science. 1999;283:854–7. doi: 10.1126/science.283.5403.854. [DOI] [PubMed] [Google Scholar]
- Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrobial agents and chemotherapy. 2003;47:833–6. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 update. World Health Organization; 2011. [PubMed] [Google Scholar]
- Gupta AK, Chauhan DS, Srivastava K, Das R, Batra S, Mittal M, Goswami P, Singhal N, Sharma VD, Venkatesan K, Hasnain SE, Katoch VM. Estimation of efflux mediated multi-drug resistance and its correlation with expression levels of two major efflux pumps in mycobacteria. The Journal of communicable diseases. 2006;38:246–54. [PubMed] [Google Scholar]
- Gupta AK, Katoch VM, Chauhan DS, Sharma R, Singh M, Venkatesan K, Sharma VD. Microarray analysis of efflux pump genes in multidrug-resistant Mycobacterium tuberculosis during stress induced by common anti-tuberculous drugs. Microbial drug resistance. 2010;16:21–8. doi: 10.1089/mdr.2009.0054. 10.1089/mdr.2009.0054. [DOI] [PubMed] [Google Scholar]
- Hagman KE, Pan W, Spratt BG, Balthazar JT, Judd RC, Shafer WM. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology. 1995;141(Pt 3):611–22. doi: 10.1099/13500872-141-3-611. [DOI] [PubMed] [Google Scholar]
- Hao P, Shi-Liang Z, Ju L, Ya-Xin D, Biao H, Xu W, Min-Tao H, Shou-Gang K, Ke W. The role of ABC efflux pump, Rv1456c-Rv1457c-Rv1458c, from Mycobacterium tuberculosis clinical isolates in China. Folia microbiologica. 2011;56:549–53. doi: 10.1007/s12223-011-0080-7. 10.1007/s12223-011-0080-7. [DOI] [PubMed] [Google Scholar]
- Helling RB, Janes BK, Kimball H, Tran T, Bundesmann M, Check P, Phelan D, Miller C. Toxic waste disposal in Escherichia coli. Journal of bacteriology. 2002;184:3699–703. doi: 10.1128/JB.184.13.3699-3703.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clinical pharmacology and therapeutics. 2005;78:260–77. doi: 10.1016/j.clpt.2005.05.011. 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Hobby GL, Meyer K, Chaffee E. Observations on the mechanism of action of penicillin. Proceedings of the Society for Experimental Biology and Medicine. 1942;50:281–288. [Google Scholar]
- Jerse AE, Sharma ND, Simms AN, Crow ET, Snyder LA, Shafer WM. A gonococcal efflux pump system enhances bacterial survival in a female mouse model of genital tract infection. Infection and immunity. 2003;71:5576–82. doi: 10.1128/IAI.71.10.5576-5582.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Zhang W, Zhang Y, Gao F, Lu C, Zhang X, Wang H. Assessment of efflux pump gene expression in a clinical isolate Mycobacterium tuberculosis by real-time reverse transcription PCR. Microbial drug resistance. 2008;14:7–11. doi: 10.1089/mdr.2008.0772. 10.1089/mdr.2008.0772. [DOI] [PubMed] [Google Scholar]
- Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. The American review of respiratory disease. 1980;121:939–49. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Hadad DJ, Dietze R, Maciel EL, Sewali B, Gitta P, Okwera A, Mugerwa RD, Alcaneses MR, Quelapio MI, Tupasi TE, Horter L, Debanne SM, Eisenach KD, Boom WH. Shortening treatment in adults with noncavitary tuberculosis and 2-month culture conversion. American journal of respiratory and critical care medicine. 2009;180:558–63. doi: 10.1164/rccm.200904-0536OC. 10.1164/rccm.200904-0536OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Join-Lambert OF, Michea-Hamzehpour M, Kohler T, Chau F, Faurisson F, Dautrey S, Vissuzaine C, Carbon C, Pechere J. Differential selection of multidrug efflux mutants by trovafloxacin and ciprofloxacin in an experimental model of Pseudomonas aeruginosa acute pneumonia in rats. Antimicrobial agents and chemotherapy. 2001;45:571–6. doi: 10.1128/AAC.45.2.571-576.2001. 10.1128/AAC.45.2.571-576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz GW. Bacterial efflux pump inhibition. Current opinion in investigational drugs. 2005;6:191–8. [PubMed] [Google Scholar]
- Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, Koul S, Khan IA. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. The Journal of antimicrobial chemotherapy. 2012 doi: 10.1093/jac/dks232. 10.1093/jac/dks232. [DOI] [PubMed] [Google Scholar]
- Klyachko KA, Schuldiner S, Neyfakh AA. Mutations affecting substrate specificity of the Bacillus subtilis multidrug transporter Bmr. Journal of bacteriology. 1997;179:2189–93. doi: 10.1128/jb.179.7.2189-2193.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler T, van Delden C, Curty LK, Hamzehpour MM, Pechere JC. Overexpression of the MexEF-OprN multidrug efflux system affects cell-to-cell signaling in Pseudomonas aeruginosa. Journal of bacteriology. 2001;183:5213–22. doi: 10.1128/JB.183.18.5213-5222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D, Peschel A. Molecular mechanisms of bacterial resistance to antimicrobial peptides. Current topics in microbiology and immunology. 2006;306:231–50. doi: 10.1007/3-540-29916-5_9. [DOI] [PubMed] [Google Scholar]
- Kumar D, Rao KV. Regulation between survival, persistence, and elimination of intracellular mycobacteria: a nested equilibrium of delicate balances. Microbes and infection / Institut Pasteur. 2011;13:121–33. doi: 10.1016/j.micinf.2010.10.009. 10.1016/j.micinf.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kupferwasser LI, Skurray RA, Brown MH, Firth N, Yeaman MR, Bayer AS. Plasmid-mediated resistance to thrombin-induced platelet microbicidal protein in staphylococci: role of the qacA locus. Antimicrobial agents and chemotherapy. 1999;43:2395–9. doi: 10.1128/aac.43.10.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche MG, Deziel E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline) PloS one. 2011;6:e24310. doi: 10.1371/journal.pone.0024310. 10.1371/journal.pone.0024310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane G, Tyagi S, Bishai WR. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infection and immunity. 2005;73:2533–40. doi: 10.1128/IAI.73.4.2533-2540.2005. 10.1128/IAI.73.4.2533-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamrabet O, Mba Medie F, Drancourt M. Acanthamoeba polyphaga-enhanced growth of Mycobacterium smegmatis. PloS one. 2012;7:e29833. doi: 10.1371/journal.pone.0029833. 10.1371/journal.pone.0029833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C, Luna B, Ammerman NC, Maiga M, Agarwal N, Bishai WR. Gene Expression of Mycobacterium tuberculosis Putative Transcription Factors whiB1-7 in Redox Environments. PloS one. 2012;7:e37516. doi: 10.1371/journal.pone.0037516. 10.1371/journal.pone.0037516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Mao W, Warren MS, Mistry A, Hoshino K, Okumura R, Ishida H, Lomovskaya O. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. Journal of bacteriology. 2000;182:3142–50. doi: 10.1128/jb.182.11.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner I, Nemeth J, Feurstein T, Abrahim A, Matzneller P, Lagler H, Erker T, Langer O, Zeitlinger M. The third-generation P-glycoprotein inhibitor tariquidar may overcome bacterial multidrug resistance by increasing intracellular drug concentration. The Journal of antimicrobial chemotherapy. 2011;66:834–9. doi: 10.1093/jac/dkq526. 10.1093/jac/dkq526. [DOI] [PubMed] [Google Scholar]
- Lewis K. Persister cells. Annual review of microbiology. 2010;64:357–72. doi: 10.1146/annurev.micro.112408.134306. 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69:1555–623. doi: 10.2165/11317030-000000000-00000. 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy. 1995;39:1948–53. doi: 10.1128/aac.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Martinez A. Effect of efflux pump inhibitors on bile resistance and in vivo colonization of Campylobacter jejuni. The Journal of antimicrobial chemotherapy. 2006;58:966–72. doi: 10.1093/jac/dkl374. 10.1093/jac/dkl374. [DOI] [PubMed] [Google Scholar]
- Lin J, Sahin O, Michel LO, Zhang Q. Critical role of multidrug efflux pump CmeABC in bile resistance and in vivo colonization of Campylobacter jejuni. Infection and immunity. 2003;71:4250–9. doi: 10.1128/IAI.71.8.4250-4259.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares JF, Lopez JA, Camafeita E, Albar JP, Rojo F, Martinez JL. Overexpression of the multidrug efflux pumps MexCD-OprJ and MexEF-OprN is associated with a reduction of type III secretion in Pseudomonas aeruginosa. Journal of bacteriology. 2005;187:1384–91. doi: 10.1128/JB.187.4.1384-1391.2005. 10.1128/JB.187.4.1384-1391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linell F, Norden A. Mycobacterium balnei, a new acid fast bacillus occurring in swimming pools and capable of producing skin lesions in humans. Acta Tuberc Scand Suppl. 1954;33:1–84. [PubMed] [Google Scholar]
- Liu J, Takiff HE, Nikaido H. Active efflux of fluoroquinolones in Mycobacterium smegmatis mediated by LfrA, a multidrug efflux pump. Journal of bacteriology. 1996;178:3791–5. doi: 10.1128/jb.178.13.3791-3795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. Journal of immunology. 2007;179:2060–3. doi: 10.4049/jimmunol.179.4.2060. [DOI] [PubMed] [Google Scholar]
- Long Q, Zhou Q, Ji L, Wu J, Wang W, Xie J. Mycobacterium smegmatis genomic characteristics associated with its saprophyte lifestyle. Journal of cellular biochemistry. 2012 doi: 10.1002/jcb.24199. 10.1002/jcb.24199. [DOI] [PubMed] [Google Scholar]
- Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Hernandez-Pando R, McEvoy CR, Grobbelaar M, Murray M, van Helden PD, Victor TC. Rifampicin Reduces Susceptibility to Ofloxacin in Rifampicin-resistant Mycobacterium tuberculosis through Efflux. American journal of respiratory and critical care medicine. 2011;184:269–76. doi: 10.1164/rccm.201011-1924OC. 10.1164/rccm.201011-1924OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louw GE, Warren RM, Gey van Pittius NC, McEvoy CR, Van Helden PD, Victor TC. A balancing act: efflux/influx in mycobacterial drug resistance. Antimicrobial agents and chemotherapy. 2009;53:3181–9. doi: 10.1128/AAC.01577-08. 10.1128/AAC.01577-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Cook DN, Alberti M, Pon NG, Nikaido H, Hearst JE. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Molecular microbiology. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Machado D, Couto I, Perdigao J, Rodrigues L, Portugal I, Baptista P, Veigas B, Amaral L, Viveiros M. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PloS one. 2012;7:e34538. doi: 10.1371/journal.pone.0034538. 10.1371/journal.pone.0034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th. Churchill Livingstone/Elsevier; Philadelphia, PA: 2010. [Google Scholar]
- Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87:1137–47. doi: 10.1016/j.biochi.2005.04.012. 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Martinez A, Lin J. Effect of an efflux pump inhibitor on the function of the multidrug efflux pump CmeABC and antimicrobial resistance in Campylobacter. Foodborne pathogens and disease. 2006;3:393–402. doi: 10.1089/fpd.2006.3.393. 10.1089/fpd.2006.3.393. [DOI] [PubMed] [Google Scholar]
- Martins M, Viveiros M, Amaral L. Inhibitors of Ca2+ and K+ transport enhance intracellular killing of M. tuberculosis by non-killing macrophages. In vivo. 2008;22:69–75. [PubMed] [Google Scholar]
- McCune RM, Jr, Tompsett R. Fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. I. The persistence of drug-susceptible tubercle bacilli in the tissues despite prolonged antimicrobial therapy. The Journal of experimental medicine. 1956;104:737–62. doi: 10.1084/jem.104.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurry L, Petrucci RE, Jr, Levy SB. Active efflux of tetracycline encoded by four genetically different tetracycline resistance determinants in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:3974–7. doi: 10.1073/pnas.77.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medjahed H, Gaillard JL, Reyrat JM. Mycobacterium abscessus: a new player in the mycobacterial field. Trends in microbiology. 2010;18:117–23. doi: 10.1016/j.tim.2009.12.007. 10.1016/j.tim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Mitchison D, Davies G. The chemotherapy of tuberculosis: past, present and future. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2012;16:724–32. doi: 10.5588/ijtld.12.0083. 10.5588/ijtld.12.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molle V, Soulat D, Jault JM, Grangeasse C, Cozzone AJ, Prost JF. Two FHA domains on an ABC transporter, Rv1747, mediate its phosphorylation by PknF, a Ser/Thr protein kinase from Mycobacterium tuberculosis. FEMS microbiology letters. 2004;234:215–23. doi: 10.1016/j.femsle.2004.03.033. 10.1016/j.femsle.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Nessar R, Cambau E, Reyrat JM, Murray A, Gicquel B. Mycobacterium abscessus: a new antibiotic nightmare. The Journal of antimicrobial chemotherapy. 2012;67:810–8. doi: 10.1093/jac/dkr578. 10.1093/jac/dkr578. [DOI] [PubMed] [Google Scholar]
- Neyfakh AA. Mystery of multidrug transporters: the answer can be simple. Molecular microbiology. 2002;44:1123–30. doi: 10.1046/j.1365-2958.2002.02965.x. [DOI] [PubMed] [Google Scholar]
- Nishino K, Latifi T, Groisman EA. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Molecular microbiology. 2006;59:126–41. doi: 10.1111/j.1365-2958.2005.04940.x. 10.1111/j.1365-2958.2005.04940.x. [DOI] [PubMed] [Google Scholar]
- Ordonez E, Letek M, Valbuena N, Gil JA, Mateos LM. Analysis of genes involved in arsenic resistance in Corynebacterium glutamicum ATCC 13032. Applied and environmental microbiology. 2005;71:6206–15. doi: 10.1128/AEM.71.10.6206-6215.2005. 10.1128/AEM.71.10.6206-6215.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla E, Llobet E, Domenech-Sanchez A, Martinez-Martinez L, Bengoechea JA, Alberti S. Klebsiella pneumoniae AcrAB efflux pump contributes to antimicrobial resistance and virulence. Antimicrobial agents and chemotherapy. 2010;54:177–83. doi: 10.1128/AAC.00715-09. 10.1128/AAC.00715-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages JM, Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochimica et biophysica acta. 2009;1794:826–33. doi: 10.1016/j.bbapap.2008.12.011. 10.1016/j.bbapap.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Parker D, Prince A. Innate immunity in the respiratory epithelium. American journal of respiratory cell and molecular biology. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca MR, Guglierame P, Arcesi F, Bellinzoni M, De Rossi E, Riccardi G. Rv2686c-Rv2687c-Rv2688c, an ABC fluoroquinolone efflux pump in Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2004;48:3175–8. doi: 10.1128/AAC.48.8.3175-3178.2004. 10.1128/AAC.48.8.3175-3178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasca MR, Guglierame P, De Rossi E, Zara F, Riccardi G. mmpL7 gene of Mycobacterium tuberculosis is responsible for isoniazid efflux in Mycobacterium smegmatis. Antimicrobial agents and chemotherapy. 2005;49:4775–7. doi: 10.1128/AAC.49.11.4775-4777.2005. 10.1128/AAC.49.11.4775-4777.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez A, Poza M, Fernandez A, Fernandez Mdel C, Mallo S, Merino M, Rumbo-Feal S, Cabral MP, Bou G. Involvement of the AcrAB-TolC efflux pump in the resistance, fitness, and virulence of Enterobacter cloacae. Antimicrobial agents and chemotherapy. 2012;56:2084–90. doi: 10.1128/AAC.05509-11. 10.1128/AAC.05509-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clinical microbiology reviews. 2006a;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nature reviews. Microbiology. 2006b;4:629–36. doi: 10.1038/nrmicro1464. 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- Pierre-Audigier C, Jouanguy E, Lamhamedi S, Altare F, Rauzier J, Vincent V, Canioni D, Emile JF, Fischer A, Blanche S, Gaillard JL, Casanova JL. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon gamma receptor deficiency. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997;24:982–4. doi: 10.1093/clinids/24.5.982. [DOI] [PubMed] [Google Scholar]
- Platz GJ, Bublitz DC, Mena P, Benach JL, Furie MB, Thanassi DG. A tolC mutant of Francisella tularensis is hypercytotoxic compared to the wild type and elicits increased proinflammatory responses from host cells. Infection and immunity. 2010;78:1022–31. doi: 10.1128/IAI.00992-09. 10.1128/IAI.00992-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas DM, Martin FA, Sabio y Garcia JV, Spera JM, Delpino MV, Baldi P, Campos E, Cravero SL, Zorreguieta A. The TolC homologue of Brucella suis is involved in resistance to antimicrobial compounds and virulence. Infection and immunity. 2007;75:379–89. doi: 10.1128/IAI.01349-06. 10.1128/IAI.01349-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillin SJ, Schwartz KT, Leber JH. The novel Listeria monocytogenes bile sensor BrtA controls expression of the cholic acid efflux pump MdrT. Molecular microbiology. 2011;81:129–42. doi: 10.1111/j.1365-2958.2011.07683.x. 10.1111/j.1365-2958.2011.07683.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Federspiel NA, Falkow S. Granuloma-specific expression of Mycobacterium virulence proteins from the glycine-rich PE-PGRS family. Science. 2000;288:1436–9. doi: 10.1126/science.288.5470.1436. [DOI] [PubMed] [Google Scholar]
- Ramon-Garcia S, Martin C, Thompson CJ, Ainsa JA. Role of the Mycobacterium tuberculosis P55 efflux pump in intrinsic drug resistance, oxidative stress responses, and growth. Antimicrobial agents and chemotherapy. 2009;53:3675–82. doi: 10.1128/AAC.00550-09. 10.1128/AAC.00550-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy TB, Riley R, Wymore F, Montgomery P, DeCaprio D, Engels R, Gellesch M, Hubble J, Jen D, Jin H, Koehrsen M, Larson L, Mao M, Nitzberg M, Sisk P, Stolte C, Weiner B, White J, Zachariah ZK, Sherlock G, Galagan JE, Ball CA, Schoolnik GK. TB database: an integrated platform for tuberculosis research. Nucleic acids research. 2009;37:D499–508. doi: 10.1093/nar/gkn652. 10.1093/nar/gkn652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8327–32. doi: 10.1073/pnas.0503272102. 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter E, Rusch-Gerdes S, Hillemann D. First linezolid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2007;51:1534–6. doi: 10.1128/AAC.01113-06. 10.1128/AAC.01113-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoll F, Pasek S, Schenowitz C, Dossat C, Barbe V, Rottman M, Macheras E, Heym B, Herrmann JL, Daffe M, Brosch R, Risler JL, Gaillard JL. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PloS one. 2009;4:e5660. doi: 10.1371/journal.pone.0005660. 10.1371/journal.pone.0005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues L, Ainsa JA, Amaral L, Viveiros M. Inhibition of drug efflux in mycobacteria with phenothiazines and other putative efflux inhibitors. Recent patents on anti-infective drug discovery. 2011a;6:118–27. doi: 10.2174/157489111796064579. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Machado D, Couto I, Amaral L, Viveiros M. Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2011b doi: 10.1016/j.meegid.2011.08.009. 10.1016/j.meegid.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Machado D, Couto I, Amaral L, Viveiros M. Contribution of efflux activity to isoniazid resistance in the Mycobacterium tuberculosis complex. Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2012;12:695–700. doi: 10.1016/j.meegid.2011.08.009. 10.1016/j.meegid.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Rodrigues LC, Lockwood D. Leprosy now: epidemiology, progress, challenges, and research gaps. The Lancet infectious diseases. 2011;11:464–70. doi: 10.1016/S1473-3099(11)70006-8. 10.1016/S1473-3099(11)70006-8. [DOI] [PubMed] [Google Scholar]
- Rohde KH, Veiga DF, Caldwell S, Balazsi G, Russell DG. Linking the Transcriptional Profiles and the Physiological States of Mycobacterium tuberculosis during an Extended Intracellular Infection. PLoS pathogens. 2012;8:e1002769. doi: 10.1371/journal.ppat.1002769. 10.1371/journal.ppat.1002769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg EY, Bertenthal D, Nilles ML, Bertrand KP, Nikaido H. Bile salts and fatty acids induce the expression of Escherichia coli AcrAB multidrug efflux pump through their interaction with Rob regulatory protein. Molecular microbiology. 2003;48:1609–19. doi: 10.1046/j.1365-2958.2003.03531.x. [DOI] [PubMed] [Google Scholar]
- Saier MH, Jr, Yen MR, Noto K, Tamang DG, Elkan C. The Transporter Classification Database: recent advances. Nucleic acids research. 2009;37:D274–8. doi: 10.1093/nar/gkn862. 10.1093/nar/gkn862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:12989–94. doi: 10.1073/pnas.2134250100. 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer WB. The effect of isoniazid on growing and resting tubercle bacilli. American review of tuberculosis. 1954;69:125–7. doi: 10.1164/art.1954.69.1.125. [DOI] [PubMed] [Google Scholar]
- Schnappinger D, Ehrt S, Voskuil MI, Liu Y, Mangan JA, Monahan IM, Dolganov G, Efron B, Butcher PD, Nathan C, Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198:693–704. doi: 10.1084/jem.20030846. 10.1084/jem.20030846 jem.20030846 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer WM, Balthazar JT, Hagman KE, Morse SA. Missense mutations that alter the DNA-binding domain of the MtrR protein occur frequently in rectal isolates of Neisseria gonorrhoeae that are resistant to faecal lipids. Microbiology. 1995;141(Pt 4):907–11. doi: 10.1099/13500872-141-4-907. [DOI] [PubMed] [Google Scholar]
- Shafer WM, Qu X, Waring AJ, Lehrer RI. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:1829–33. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kumar M, Nargotra A, Koul S, Khan IA. Piperine as an inhibitor of Rv1258c, a putative multidrug efflux pump of Mycobacterium tuberculosis. The Journal of antimicrobial chemotherapy. 2010;65:1694–701. doi: 10.1093/jac/dkq186. 10.1093/jac/dkq186. [DOI] [PubMed] [Google Scholar]
- Shepard CC. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. The Journal of experimental medicine. 1957;105:39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi N, Das R, Pathak N, Banerjee S, Ahmed N, Katoch VM, Hasnain SE. Mycobacterium tuberculosis isolate with a distinct genomic identity overexpresses a tap-like efflux pump. Infection. 2004;32:109–11. doi: 10.1007/s15010-004-3097-x. 10.1007/s15010-004-3097-x. [DOI] [PubMed] [Google Scholar]
- Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L, Brosch R, Enninga J. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS pathogens. 2012;8:e1002507. doi: 10.1371/journal.ppat.1002507. 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Jadaun GP, Ramdas, Srivastava K, Chauhan V, Mishra R, Gupta K, Nair S, Chauhan DS, Sharma VD, Venkatesan K, Katoch VM. Effect of efflux pump inhibitors on drug susceptibility of ofloxacin resistant Mycobacterium tuberculosis isolates. The Indian journal of medical research. 2011;133:535–40. [PMC free article] [PubMed] [Google Scholar]
- Singh P, Cole ST. Mycobacterium leprae: genes, pseudogenes and genetic diversity. Future microbiology. 2011;6:57–71. doi: 10.2217/fmb.10.153. 10.2217/fmb.10.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spies FS, da Silva PE, Ribeiro MO, Rossetti ML, Zaha A. Identification of mutations related to streptomycin resistance in clinical isolates of Mycobacterium tuberculosis and possible involvement of efflux mechanism. Antimicrobial agents and chemotherapy. 2008;52:2947–9. doi: 10.1128/AAC.01570-07. 10.1128/AAC.01570-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivey VL, Molle V, Whalan RH, Rodgers A, Leiba J, Stach L, Walker KB, Smerdon SJ, Buxton RS. Forkhead-associated (FHA) domain containing ABC transporter Rv1747 is positively regulated by Ser/Thr phosphorylation in Mycobacterium tuberculosis. The Journal of biological chemistry. 2011;286:26198–209. doi: 10.1074/jbc.M111.246132. 10.1074/jbc.M111.246132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Critical reviews in food science and nutrition. 2007;47:735–48. doi: 10.1080/10408390601062054. 10.1080/10408390601062054. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Musuka S, Sherman C, Meek C, Leff R, Gumbo T. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. The Journal of infectious diseases. 2010;201:1225–31. doi: 10.1086/651377. 10.1086/651377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL, Roth R, Takeshita S, Heuser J, Welch MD, Brown EJ. Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. The Journal of experimental medicine. 2003;198:1361–8. doi: 10.1084/jem.20031072. 10.1084/jem.20031072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinear TP, Seemann T, Harrison PF, Jenkin GA, Davies JK, Johnson PD, Abdellah Z, Arrowsmith C, Chillingworth T, Churcher C, Clarke K, Cronin A, Davis P, Goodhead I, Holroyd N, Jagels K, Lord A, Moule S, Mungall K, Norbertczak H, Quail MA, Rabbinowitsch E, Walker D, White B, Whitehead S, Small PL, Brosch R, Ramakrishnan L, Fischbach MA, Parkhill J, Cole ST. Insights from the complete genome sequence of Mycobacterium marinum on the evolution of Mycobacterium tuberculosis. Genome research. 2008;18:729–41. doi: 10.1101/gr.075069.107. 10.1101/gr.075069.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BJ, Miller VL. Salmonella enteritidis has a homologue of tolC that is required for virulence in BALB/c mice. Molecular microbiology. 1995;17:701–12. doi: 10.1111/j.1365-2958.1995.mmi_17040701.x. [DOI] [PubMed] [Google Scholar]
- Taylor DL, Bina XR, Bina JE. Vibrio cholerae VexH encodes a multiple drug efflux pump that contributes to the production of cholera toxin and the toxin co-regulated pilus. PloS one. 2012;7:e38208. doi: 10.1371/journal.pone.0038208. 10.1371/journal.pone.0038208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekaia F, Gordon SV, Garnier T, Brosch R, Barrell BG, Cole ST. Analysis of the proteome of Mycobacterium tuberculosis in silico. Tuber Lung Dis. 1999;79:329–42. doi: 10.1054/tuld.1999.0220. [DOI] [PubMed] [Google Scholar]
- Thanassi DG, Cheng LW, Nikaido H. Active efflux of bile salts by Escherichia coli. Journal of bacteriology. 1997;179:2512–8. doi: 10.1128/jb.179.8.2512-2518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. Properties of Mycobacterium smegmatis freshly isolated from soil. Japanese journal of microbiology. 1976;20:355–6. doi: 10.1111/j.1348-0421.1976.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Tzeng YL, Ambrose KD, Zughaier S, Zhou X, Miller YK, Shafer WM, Stephens DS. Cationic antimicrobial peptide resistance in Neisseria meningitidis. Journal of bacteriology. 2005;187:5387–96. doi: 10.1128/JB.187.15.5387-5396.2005. 10.1128/JB.187.15.5387-5396.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M, Pierson J, Brenner M, Peters PJ. M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell. 2007;129:1287–98. doi: 10.1016/j.cell.2007.05.059. 10.1016/j.cell.2007.05.059. [DOI] [PubMed] [Google Scholar]
- Viveiros M, Portugal I, Bettencourt R, Victor TC, Jordaan AM, Leandro C, Ordway D, Amaral L. Isoniazid-induced transient high-level resistance in Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2002;46:2804–10. doi: 10.1128/AAC.46.9.2804-2810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RJ, Jr, Nash DR, Tsukamura M, Blacklock ZM, Silcox VA. Human disease due to Mycobacterium smegmatis. The Journal of infectious diseases. 1988;158:52–9. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- Wallis RS, Patil S, Cheon SH, Edmonds K, Phillips M, Perkins MD, Joloba M, Namale A, Johnson JL, Teixeira L, Dietze R, Siddiqi S, Mugerwa RD, Eisenach K, Ellner JJ. Drug tolerance in Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 1999;43:2600–6. doi: 10.1128/aac.43.11.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006;367:1849–58. doi: 10.1016/S0140-6736(06)68807-7. 10.1016/S0140-6736(06)68807-7. [DOI] [PubMed] [Google Scholar]
- Ward SK, Abomoelak B, Hoye EA, Steinberg H, Talaat AM. CtpV: a putative copper exporter required for full virulence of Mycobacterium tuberculosis. Molecular microbiology. 2010;77:1096–110. doi: 10.1111/j.1365-2958.2010.07273.x. 10.1111/j.1365-2958.2010.07273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DM, Levy SB. Different effects of transcriptional regulators MarA, SoxS and Rob on susceptibility of Escherichia coli to cationic antimicrobial peptides (CAMPs): Rob-dependent CAMP induction of the marRAB operon. Microbiology. 2010;156:570–8. doi: 10.1099/mic.0.033415-0. 10.1099/mic.0.033415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DM, Shafer WM, Jerse AE. Clinically relevant mutations that cause derepression of the Neisseria gonorrhoeae MtrC-MtrD-MtrE Efflux pump system confer different levels of antimicrobial resistance and in vivo fitness. Molecular microbiology. 2008;70:462–78. doi: 10.1111/j.1365-2958.2008.06424.x. 10.1111/j.1365-2958.2008.06424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ, Iavarone AT, Portnoy DA. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–5. doi: 10.1126/science.1189801. 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolridge DP, Vazquez-Laslop N, Markham PN, Chevalier MS, Gerner EW, Neyfakh AA. Efflux of the natural polyamine spermidine facilitated by the Bacillus subtilis multidrug transporter Blt. The Journal of biological chemistry. 1997;272:8864–6. doi: 10.1074/jbc.272.14.8864. [DOI] [PubMed] [Google Scholar]
- Wu Y, Vulic M, Keren I, Lewis K. Role of Oxidative Stress in Persister Tolerance. Antimicrobial agents and chemotherapy. 2012 doi: 10.1128/AAC.00921-12. 10.1128/AAC.00921-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Danelishvili L, Wu M, Hidaka E, Katsuyama T, Stang B, Petrofsky M, Bildfell R, Bermudez LE. The ability to form biofilm influences Mycobacterium avium invasion and translocation of bronchial epithelial cells. Cellular microbiology. 2006;8:806–14. doi: 10.1111/j.1462-5822.2005.00667.x. 10.1111/j.1462-5822.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- Yanong RP, Pouder DB, Falkinham JO., 3rd Association of mycobacteria in recirculating aquaculture systems and mycobacterial disease in fish. Journal of aquatic animal health. 2010;22:219–23. doi: 10.1577/H10-009.1. 10.1577/H10-009.1. [DOI] [PubMed] [Google Scholar]
- Zahner D, Zhou X, Chancey ST, Pohl J, Shafer WM, Stephens DS. Human antimicrobial peptide LL-37 induces MefE/Mel-mediated macrolide resistance in Streptococcus pneumoniae. Antimicrobial agents and chemotherapy. 2010;54:3516–9. doi: 10.1128/AAC.01756-09. 10.1128/AAC.01756-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Yue J, Yang YP, Zhang HM, Lei JQ, Jin RL, Zhang XL, Wang HH. Detection of mutations associated with isoniazid resistance in Mycobacterium tuberculosis isolates from China. Journal of clinical microbiology. 2005;43:5477–82. doi: 10.1128/JCM.43.11.5477-5482.2005. 10.1128/JCM.43.11.5477-5482.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierski M, Bek E, Long MW, Snider DE., Jr Short-course (6 month) cooperative tuberculosis study in Poland: results 18 months after completion of treatment. The American review of respiratory disease. 1980;122:879–89. doi: 10.1164/arrd.1980.122.6.879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


