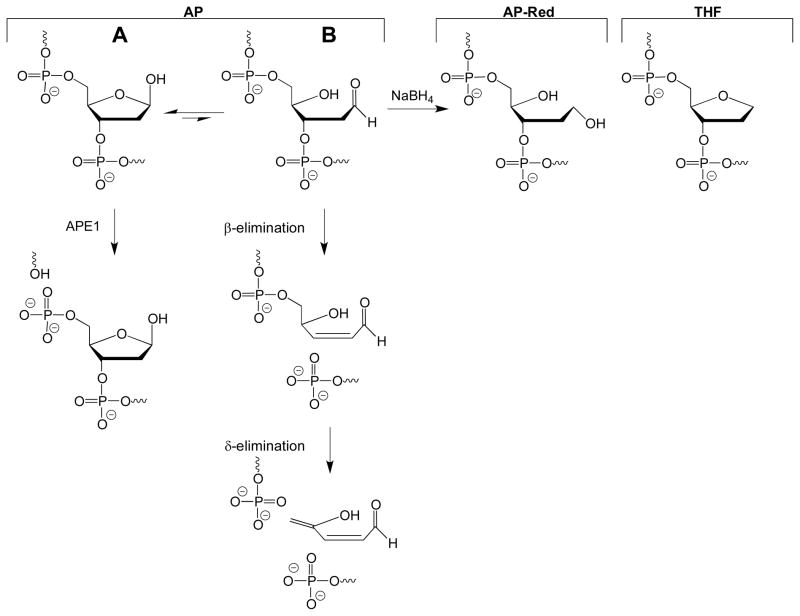
FIGURE 1.
Structures of AP, AP-Red, and THF. The authentic AP site exists as an equilibrium between the ring-closed hemiacetal (A) and ring-opened aldehyde (B). The ring-opened aldehyde is subject to β-elimination, and can further be converted to the β,δ-elimination product under basic conditions. The ring-opened aldehyde can be reduced by NaBH4 to generate the AP-Red substrate. APE1 incises the AP site, to create a nick with a 3′-OH and 5′-dRP group.