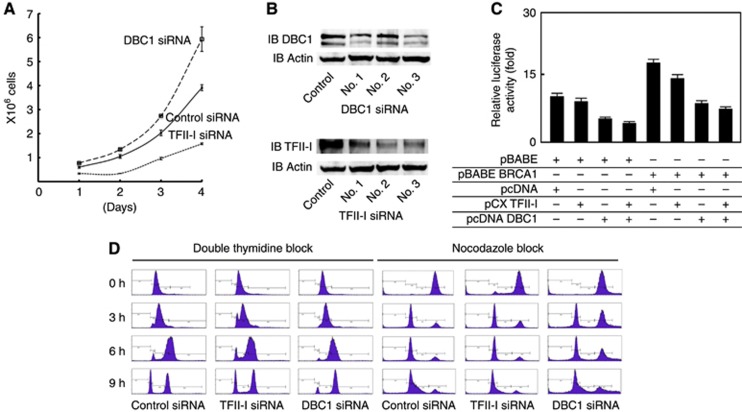
Figure 1.
The cell-cycle regulation by TFII-I and DBC1. (A) Trypan blue dye exclusion test was performed to examine the effect on cellular growth in U2OS cells. In this assay, siRNA-mediated knockdown of endogenous DBC1 or TFII-I was performed and 5 × 105 U2OS cells were allowed to grow for a subsequent 3 days. The knockdown of DBC1 resulted in an increase in cell numbers compared with control siRNA 3 days after treatment, whereas the depletion of TFII-I resulted in a decrease in cell numbers compared with control. (B) Efficiency of siRNA-mediated knockdown of TFII-I and DBC1 is demonstrated by western blot. (C) Transient transfection assays were performed to examine the functions of TFII-I and DBC1 in BRCA1-mediated cell-cycle regulation. 293T cells were transfected with the indicated combinations of mammalian expression plasmids, and the transfected whole-cell lysates were assayed for luciferase activity. DBC1 repressed GADD45 promoter activity regardless of the presence of BRCA1. The error bars represent the standard deviations. (D) Flow cytometry analysis was performed using U2OS cells synchronized using a double-thymidine block. The cells were subsequently released into S phase. In cells depleted with endogenous TFII-I, increased G1–S boundary cell fraction was observed at 3–6 h after thymidine release. In cells depleted with endogenous DBC1, decreased fraction of postmitotic G1 peak was observed at 9 h after thymidine release. The cells treated with siRNA as indicated were also synchronized by a nocodazole block/release. The siRNA-mediated knockdown of DBC1 resulted in increased accumulation of the G2/M fraction 3–6 h after nocodazole release.