Abstract
Partially edentulous patients may be rehabilitated by the placement of removable dental prostheses, implant-supported removable dental prostheses, or partial implant fixed dental prostheses. However, it is unclear the impact of each prosthesis type over the masticatory aspects, which represents the objective of this paired clinical trial. Twelve patients sequentially received and used each of these 3 prosthesis types for 2 months, after which maximum bite force was assessed by a strain sensor and food comminution index was determined with the sieving method. Masseter and temporal muscle thicknesses during rest and maximal clenching were also evaluated by ultrasonography. Each maxillary arch received a new complete denture that was used throughout the study. Data were analyzed by analysis of variance for repeated measures, followed by the Tukey test (p < .05). Maximum bite force and food comminution index increased (p < .0001) after implant-supported dental prosthesis and implant fixed dental prosthesis use, with the higher improvement found after the latter’s use. Regardless of implant-retained prosthesis type, masseter muscle thickness during maximal clenching also increased (p < .05) after implant insertion. Partial implant-supported prostheses significantly improved masseter muscle thickness and mastication, and the magnitude of this effect was related to prosthesis type (International Clinical Trial Registration RBR-9J26XD).
Keywords: clinical trials, mastication, removable prosthodontics, fixed prosthodontics, oral rehabilitation, ultrasound
Introduction
Posterior teeth play important roles in comminuting food, and postcanine teeth loss significantly reduces masticatory performance (van der Bilt et al., 2006). Moreover, loss of a first-molar occlusal pair is a key factor in prosthetic restoration (Fueki et al., 2011).
Several prosthetic options are available to restore chewing function in patients with missing teeth (Abt et al., 2012; de Freitas et al., 2012). However, few studies (Kapur, 1991; Liedberg et al., 2004) have determined the effects of prosthetic treatment on mastication in partially edentulous patients, and their findings are controversial. Kapur (1991) reported that removable dental prostheses (RDPs) and partial implant fixed dental prostheses (IFDPs) achieved similar chewing efficiency. In contrast, Liedberg et al. (2004) showed higher food comminution in patients with fixed dental prostheses than in RDP wearers. Because masticatory impairment can adversely affect quality of life (Lepley et al., 2010), the effects of different prostheses on mastication are important to determine.
Several methods have been used to evaluate mastication, including occlusal force measurements (Goshima et al., 2010; Muller et al., 2012; Ohara et al., 2013), sieving test (Gotfredsen and Walls, 2007; van der Bilt, 2011), color-changeable gum test (Goshima et al., 2010; Muller et al., 2012), and muscle thickness evaluation (Bhoyar et al., 2012; Muller et al., 2012; Ohara et al., 2013). In addition, correlations among bite force, chewing performance, and masticatory muscle thickness (Raadsheer et al., 1999; Muller et al., 2012) have been established, and it is known that masticatory muscle action is influenced by occlusal factors, such as partial edentulism (Bhoyar et al., 2012). Thus, masticatory muscle function can be reduced by severe tooth loss or a soft diet consumption, as typically selected by edentulous patients, leading to muscle atrophy (Tsai et al., 2012).
Dental implants are increasingly used to replace missing teeth (Abt et al., 2012; de Freitas et al., 2012), and studies have shown masticatory improvements in implant-supported overdenture wearers (Carlsson and Lindquist, 1994; Feine et al., 1994; Geertman et al., 1999; van Kampen et al., 2004). However, the effect of implant therapy is unclear in partially edentulous patients’ chewing, which was the aim of this study. The tested hypothesis was that the increased retention and stability provided by implants would be predictive of masticatory improvements and could affect muscle thickness.
Materials & Methods
Experimental Design
The Ethics Committee of Piracicaba Dental School, University of Campinas (Piracicaba, Brazil), approved this research (protocol 011/2010). In this longitudinal, single-center clinical trial, subjects served as their own (paired) controls. Study participation was voluntary, and subjects provided written informed consent before enrollment (International Clinical Trial Registration RBR-9J26XD).
Subjects were selected with edentulous maxilla and partial edentulous mandible, using old and ill-fitting removable dentures. Each patient received a new, complete maxillary denture that was used throughout the study, while a sequence of 3 mandibular treatments was performed: conventional RDPs, implant-supported removable dental prostheses (IRDPs), and IFDPs. All treatments were accomplished with no cost to the subjects, and each prosthetic treatment was used for 2 months before masticatory evaluation. We measured the maximum bite force (MBF), food comminution index (FCI), and masticatory muscle thickness. The poor conditions of the old prostheses did not allow for masticatory evaluation at baseline.
Subject Selection
Eligible subjects had no maxillary teeth and only mandibular canines and incisors, with sufficient bone in the posterior mandible to allow for implant installation. Subjects were in good general health and free of temporomandibular disorder, parafunctional habits, or uncontrolled systemic disease that would prevent oral surgery.
Sample size was estimated on the basis of a previous study (Miyaura et al., 2000) (bidirectional α of 0.05 and β of 0.20), and 9.6 subjects were required to detect differences. We added 25% to compensate patient drawback, with a total sample of 12.
Patients seeking prosthetic treatment at Piracicaba Dental School, University of Campinas, were contacted (n = 120), but 12 were excluded because of advanced periodontal disease, 33 because of the retention of lower molars and/or premolars, and 57 because of insufficient bone height for implant insertion (evaluated by panoramic radiography and/or computed tomography). Three patients refused to participate. Thus, 15 were selected, but 1 died during the research period and 2 were excluded because of bone resorption complications, yielding a final sample of 12 volunteers (4 men, 8 women) with a mean age of 62.6 ± 7.8 yrs (range, 48-80 yrs).
Clinical Procedures
Subjects received general dental treatment, including periodontal and dental care for remaining teeth. New complete maxillary dentures and mandibular RDPs were assembled with conventional techniques. RDP frameworks were made of cobalt-chromium alloy, with lingual major bar and circumferential or bar clasp retainers as the RPD design. Lingual rests were located on the lower canine cingulum and provided indirect retention to rotational movements. Prostheses were installed and adjusted in patients’ mouths with bilateral balanced occlusion scheme. After 2 months of prosthesis use, mastication was evaluated.
Subjects received 2 implants (Titamax; Neodent, Curitiba, Brazil) per side in the mandibular premolar and molar region. The correct implant position and inclination were established with a surgical guide, and a conventional 2-step technique was used (Blanes et al., 2007). After 1 week, RDPs were adjusted and relined with resilient soft lining material (Ufi Gel P; Voco, Cuxhaven, Germany) for use during the 4-month osseointegration period.
The posterior implants were exposed and received ball abutments (O’ring; Neodent) according to the manufacturer’s instructions. Conventional RDP acrylic base was relieved, and the capsules were captured directly in the mouth to improve passive fit (de Freitas et al., 2012), transforming the RDP into an IRDP. Occlusal adjustments were performed to maintain bilateral balanced occlusion. Masticatory variables were again evaluated after 2 months of IRDP use.
At final step, IRDP was replaced by 3-unit metal-ceramic IFDP assembled with conventional techniques (Blanes et al., 2007). All IFDPs were screwed over abutments (Mini Pilar; Neodent) attached to implants, according to manufacturer’s instructions. The screw holes were covered with compound resin, and occlusal adjustment was performed. After 2 months of IFDP use, masticatory function was evaluated.
Masticatory Function Evaluation
MBF was measured with bite force transducer (Spider 8; Hottinger Baldwin Messtechnik GmbH, Darmstadt, Germany) (Fernandes et al., 2003). Sensors (FSR no. 151, 1.2-mm diameter, 5.6-mm thickness; Interlink Electronics Inc., Camarillo, CA, USA) were placed in the bilateral first molar regions, and signals were recorded and analyzed by Catman Easy software (version 1.0; Hottinger Baldwin Messtechnik GmbH). Subjects were requested to occlude with maximum force for 7 s, and the procedure was repeated after 5-min rest. The average of the 2 measurements was calculated and recorded in newtons (N).
The reproducibility of the MBF method was verified in 10 subjects chosen at random. Two measurements were performed, and a high intraclass correlation coefficient was found (r = 0.94).
FCI was evaluated with Optocal artificial test material (Pocztaruk et al., 2008). Subjects were instructed to chew a 3.7-g portion in the habitual manner for 20 strokes (van der Bilt and Fontijn-Tekamp, 2004) while a single calibrated operator counted the cycles. The comminuted particles were collected, dried, and vibrated in a sieving machine (Bertel Indústria Metalúrgica, Caieiras, Brazil) through a stack of sieves with variably sized mesh (0.5 mm to 5.6 mm). Materials retained on sieves were weighed on a 0.001-g analytical balance (Mark; BEL Engineering, Milan, Italy), and the FCI was calculated as the percentage weight of the comminuted material that passed through the 2.8-mm sieve (van der Bilt and Fontijn-Tekamp, 2004).
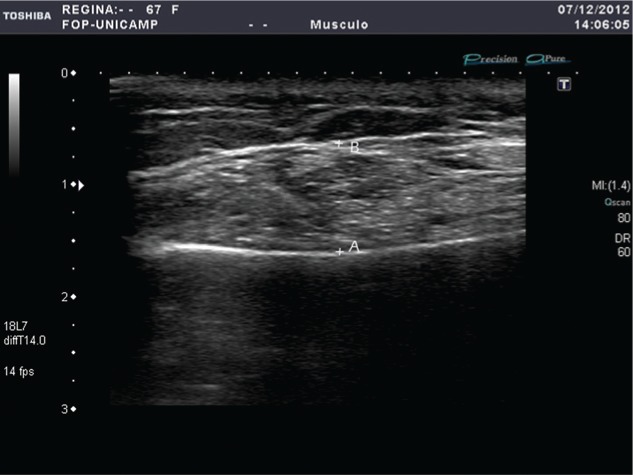
Real-time imaging of the bilateral masseter and anterior temporalis muscle thickness was performed ultrasonographically (SSA-780 A-APLIO Mx, 38 mm/7-18 MHz; Toshiba Medical System Co., Tokyo, Japan). Muscle thickness was measured directly on the instrument’s screen (Fig. 1) with an accuracy of 0.01 mm (Castelo et al., 2010).
Figure 1.
Example of an ultrasound image of masseter muscle thickness (mm) during maximum muscle contraction. The intensive white line at the lower part of the image is the echo of the lateral surface of the ramus mandibularis (A), and the narrow white line at the top represents the outer fascia of the masseter muscle (B). The masseter is seen as a dark area between the fascia (B) and the lateral surface of the ramus (A) measured perpendicular to the ramus.
A pilot study was performed on 2 days with 10 subjects selected at random. The ultrasound measurement error (Se) was calculated by Dahlberg’s (1940) formula: Se = √Σd 2/2n, where d is the difference between 2 measurements and n is the number of recordings. The masseter muscle thickness errors in contracted and relaxed positions were 0.13 and 0.16 mm, respectively, and those for the anterior temporalis were 0.17 and 0.16 mm. These values are considered small, revealing the method’s accuracy (Georgiakaki et al., 2007). Additionally, a Pearson correlation coefficient performed between the 2 measurements revealed a strong and significant correlation (r = 0.85-0.98) (p < .0001).
Each trial was conducted in a darkened room with the subject seated in an upright position. All measurements were performed by a single calibrated operator to avoid interoperator error (Emshoff et al., 2003). A standardized protocol was used to establish the correct location of the muscle site (Emshoff et al., 2003). Initially, the muscles were identified by palpation (masseter: area of greatest lateral distention, ~ 2 cm above the inferior mandibular border; anterior temporalis: anterior to the anterior border of the hairline) (Castelo et al., 2010), and a line was drawn on the subject’s skin, showing the specific area where the transducer should be placed. After gel application, the probe was held perpendicular to the muscle, avoiding excessive pressure on the tissue, until the reflection of the bone was depicted as a sharp white line. The thickest part of the muscles was measured perpendicular to the muscle long axis (Fig. 1) (Castelo et al., 2010). Three measurements were performed for each muscle at rest and in the maximum voluntary clenching (MVC). Final muscle thickness values were obtained by averaging these values (Castelo et al., 2010).
Statistical Analyses
Data distributions were assessed by Shapiro-Wilk tests, which revealed normal distributions. Analysis of variance for repeated measures was performed with SAS software (release 9.1, 2003; SAS Institute Inc., Cary, NC, USA), and Tukey-Kramer tests were used for comparisons among the prosthetic treatments. Pearson correlations were calculated among masticatory muscle thickness, MBF, and FCI. Statistical significance was set to p < .05.
Results
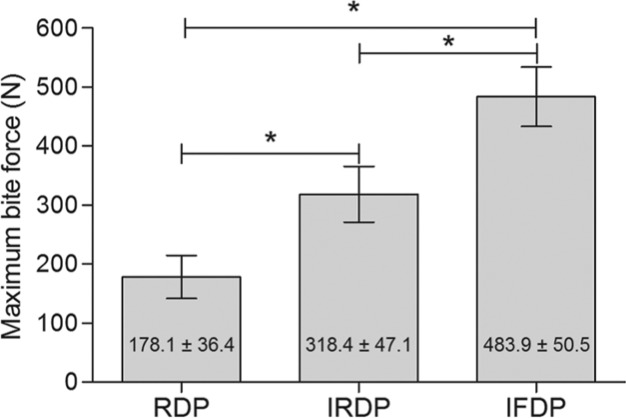
MBF increased (p < .0001) after implant insertion (Fig. 2) with gain of 140 N observed between RDP and IRDP use, while an increment of 306 N was detected from RDP to IFDP use, growing 79% and 172%, respectively.
Figure 2.
Graph showing mean value of maximum bite force (N) and standard deviations in relation to the prosthetic treatment. Maximum bite force was significantly higher for the implant-supported removable dental prosthesis (IRDP) and implant fixed dental prosthesis (IFDP) (*p < .0001). RDP, removable dental prosthesis.
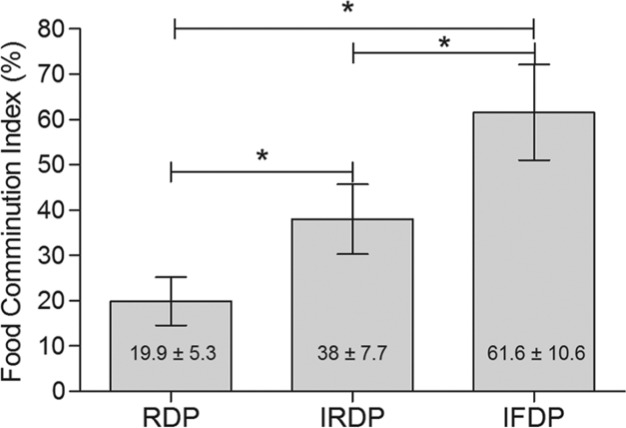
A similar trend was observed for FCI, with the highest values verified after IFDP use (p < .0001) (Fig. 3). Multiple comparisons among RDP, IRDP, and IFDP use revealed that FCI rose up to 91% when comparing RDP to IRDP use, while the improvement found between RDP and IFDP use was 209% on average.
Figure 3.
Graph showing mean value of food comminution index (%) and standard deviation in relation to the prosthetic treatment. The use of implant-supported removable dental prostheses (IRDPs) and implant fixed dental prostheses (IFDP) significantly increased the chewing capacity (*p < .0001). RDP, removable dental prosthesis.
The left and right masseter and anterior temporalis muscle thicknesses during rest and MVC are presented in the Table. Regardless of side and prosthesis type, masseter muscle thickness during MVC increased after implant insertion (p < .05), rising from 5.9% to 9.3% in respect to muscle site and prosthesis type. No differences were observed in masseter or temporalis muscle thickness at rest or temporalis muscle in MVC (all p > .05).
Table.
Masseter and Anterior Temporalis Muscle Thicknesses in Relation to Prosthesis Type, Jaw Position, and Side, mm (Mean ± Standard Deviation)
| Prostheses |
|||
|---|---|---|---|
| Muscle | Removable Dental | Implant-supported Removable | Implant Fixed Dental |
| Masseter | |||
| Right | |||
| Rest | 10.3 ± 1.6a | 10.3 ± 1.7a | 10.6 ± 1.7a |
| MVC | 11.8 ± 1.5a | 12.5 ± 1.3b | 12.8 ± 1.4b |
| Left | |||
| Rest | 10 ± 1.4a | 10.3 ± 1.7a | 10.3 ± 1.9a |
| MVC | 11.8 ± 1.9a | 12.5 ± 2b | 12.9 ± 2b |
| Anterior temporal | |||
| Right | |||
| Rest | 3.2 ± 0.8a | 3.3 ± 0.7a | 3.4 ± 0.7a |
| MVC | 4.2 ± 0.9a | 4.3 ± 0.9a | 4.3 ± 1a |
| Left | |||
| Rest | 3.2 ± 0.7a | 3.2 ± 0.7a | 3.3 ± 1a |
| MVC | 4.1 ± 0.8a | 4.2 ± 0.7a | 4.2 ± 0.8a |
MVC = maximum voluntary clenching. a,bDifferent letters indicate significant differences among treatments. Repeated measures analysis of variance, Tukey honestly significant difference, p < .05.
Pearson correlation analysis performed between muscle thicknesses and masticatory variables revealed weak and nonsignificant correlations (p > .05).
Discussion
Given the common occurrence of tooth loss, increasing life spans, and retention of more teeth into advanced age, evidence is needed to inform the clinical management of tooth loss (Abt et al., 2012). Studies comparing different prostheses must eliminate confounding factors (Abt et al., 2012), and these can be achieved most reliably by intraindividual comparison of restoration alternatives. This paired study provides sufficient evidences for the effects of prosthetic treatment on masticatory function in partially edentulous patients. Simple, accurate, and reliable methods were used to quantify mastication provided by each dental restorative procedure.
As expected, MBF was higher after IFDP and IRDP use than after RDP use. Although no other paired study on this topic has been published, our MBF findings are in accordance with those of Miyaura et al. (2000) and Ohara et al. (2013). Nevertheless, greater bite forces are associated with higher masticatory capacity (Lepley et al., 2010), as confirmed by the FCI results of the present study. Previous studies (Carlsson and Lindquist, 1994; Feine et al., 1994; Geertman et al., 1999; van Kampen et al., 2004) with similar methodologies also agree with these results, although they had evaluated completely edentulous patients. In contrast, Kapur (1991) revealed no difference in mastication between RDP and IFDP wearers; however, this similarity might be due to the chewing platform reduction in IFDP group. Authors pointed out that this reduction was necessary to prevent damage to the blade implants system (Kapur, 1991). In our case, mandibular prosthesis occlusion was based on the nonchanged maxillary denture, keeping the chewing platform similar in all prostheses. The increased masticatory function may be related to the drastic reduction in RPD rotational movement after implant insertion, which allowed the development of stronger jaw elevator muscles (Lepley et al., 2010), increasing the ability to comminute test material. It is important to highlight the advantages of IRDP therapy compared to IFDP in relation to the reduced cost and small amount of implants needed (de Freitas et al., 2012). Therefore, IRDP therapy properly restores masticatory function of partially edentulous patients, representing a reliable and more affordable treatment to be offered in the clinical routine.
MBF is considered a key factor of masticatory function (Muller et al., 2012), and masseter muscle thickness was shown to be a major contributing factor of bite force (Raadsheer et al., 1999). Furthermore, periodontal mechanoreceptors play a key role in masticatory force control during food chewing (Trulsson, 2006; Abt et al., 2012), revealing the importance of tooth maintenance. In the present study, the effects of the implant therapy were clearly observed in both MBF and masseter muscle thickness during clenching. Similar muscle changes were observed by a previous study (Bhoyar et al., 2012) after 3-month use of new complete dentures. In addition, Tsai et al. (2012) described that the constant intake of soft food could result in masticatory muscle atrophy (Bhoyar et al., 2012; Muller et al., 2012). Thus, it could be suggested that the enlarged masseter muscle thickness may be related to the higher intake of chewy food, which requires a more vigorous action of the masticatory muscles, explaining the masseter thickness changes. Despite the differences in masseter muscle thickness during MVC, no change in muscle thickness at rest was observed, which was predictable given the short duration of each treatment. Future studies with long-term follow-up are needed to evaluate changes in masticatory muscles over time.
Although our data show a dramatic masticatory improvement after implant insertion, special attention must be given to the relatively small sample and short follow-up period. Based on the statistical estimation, it seems unlikely that increasing sample size would change the results. Nevertheless, a paired experimental design was used avoiding bias, since each subject acts as one’s own control. The short-term follow-up allowed the analysis of different treatments in the same subject without drawbacks. In addition, measurements were performed only after the complete adaptation of subjects to each prosthetic treatment, when no more chewing complaints were reported.
Mastication can be evaluated by objective and subjective methods (Gotfredsen and Walls, 2007; van der Bilt, 2011). In this study, only objective parameters of mastication were evaluated because subjective chewing assessment is, in general, too optimistic because of the great variability in tooth loss adaptation (Gotfredsen and Walls, 2007; van der Bilt, 2011). Therefore, the single-sieve method was selected because it is a convenient and reliable method to evaluate the capacity of food comminution (van der Bilt, 2011).
Our data show the real impact of different prosthetic treatments on mastication in partially edentulous patients. However, future investigations should determine the consequences of masticatory improvement on nutritional intake, swallowing threshold, chewing ability, and quality of life.
Conclusions
The IRDPs and IFDPs significantly increased MBF and FCI, with the magnitude of the masticatory improvements closely related to prosthesis type. The use of implants also increased masseter muscle thickness during contraction.
Footnotes
This study was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo a Pesquisa do Estado de São Paulo (grant 2010/12251-0), Brazil.
The authors declare no conflicts of interest with respect to the authorship and/or publication of this article.
References
- Abt E, Carr AB, Worthington HV. (2012). Interventions for replacing missing teeth: partially absent dentition. Cochrane Database Syst Rev 2:CD003814. [DOI] [PubMed] [Google Scholar]
- Bhoyar PS, Godbole SR, Thombare RU, Pakhan AJ. (2012). Effect of complete edentulism on masseter muscle thickness and changes after complete denture rehabilitation: an ultrasonographic study. J Investig Clin Dent 3:45-50 [DOI] [PubMed] [Google Scholar]
- Blanes RJ, Bernard JP, Blanes ZM, Belser UC. (2007). A 10-year prospective study of ITI dental implants placed in the posterior region: I. Clinical and radiographic results. Clin Oral Implants Res 18:699-706 [DOI] [PubMed] [Google Scholar]
- Carlsson GE, Lindquist LW. (1994). Ten-year longitudinal study of masticatory function in edentulous patients treated with fixed complete dentures on osseointegrated implants. Int J Prosthodont 7:448-453 [PubMed] [Google Scholar]
- Castelo PM, Gaviao MB, Pereira LJ, Bonjardim LR. (2010). Evaluation of changes in muscle thickness, bite force and facial asymmetry during early treatment of functional posterior crossbite. J Clin Pediatr Dent 34:369-374 [DOI] [PubMed] [Google Scholar]
- Dahlberg G. (1940). Statistical methods for medical and biological students. New York, NY: Interscience Publications [Google Scholar]
- de Freitas RF, de Carvalho Dias K, da Fonte Porto Carreiro A, Barbosa GA, Ferreira MA. (2012). Mandibular implant-supported removable partial denture with distal extension: a systematic review. J Oral Rehabil 39:791-798 [DOI] [PubMed] [Google Scholar]
- Emshoff R, Emshoff I, Rudisch A, Bertram S. (2003). Reliability and temporal variation of masseter muscle thickness measurements utilizing ultrasonography. J Oral Rehabil 30:1168-1172 [DOI] [PubMed] [Google Scholar]
- Feine JS, Maskawi K, de Grandmont P, Donohue WB, Tanguay R, Lund JP. (1994). Within-subject comparisons of implant-supported mandibular prostheses: evaluation of masticatory function. J Dent Res 73:1646-1656 [DOI] [PubMed] [Google Scholar]
- Fernandes CP, Glantz PO, Svensson SA, Bergmark A. (2003). A novel sensor for bite force determinations. Dent Mater 19:118-126 [DOI] [PubMed] [Google Scholar]
- Fueki K, Igarashi Y, Maeda Y, Baba K, Koyano K, Akagawa Y, et al. (2011). Factors related to prosthetic restoration in patients with shortened dental arches: a multicentre study. J Oral Rehabil 38:525-532 [DOI] [PubMed] [Google Scholar]
- Geertman ME, Slagter AP, van’t Hof MA, van Waas MA, Kalk W. (1999). Masticatory performance and chewing experience with implant-retained mandibular overdentures. J Oral Rehabil 26:7-13 [DOI] [PubMed] [Google Scholar]
- Georgiakaki I, Tortopidis D, Garefis P, Kiliaridis S. (2007). Ultrasonographic thickness and electromyographic activity of masseter muscle of human females. J Oral Rehabil 34:121-128 [DOI] [PubMed] [Google Scholar]
- Goshima K, Lexner MO, Thomsen CE, Miura H, Gotfredsen K, Bakke M. (2010). Functional aspects of treatment with implant-supported single crowns: a quality control study in subjects with tooth agenesis. Clin Oral Implants Res 21:108-114 [DOI] [PubMed] [Google Scholar]
- Gotfredsen K, Walls AW. (2007). What dentition assures oral function? Clin Oral Implants Res 18(suppl 3):34-45 (Published erratum in Clin Oral Implants Res 19:326-328, 2008.) [DOI] [PubMed] [Google Scholar]
- Kapur KK. (1991). Veterans Administration Cooperative Dental Implant Study: comparisons between fixed partial dentures supported by blade-vent implants and removable partial dentures. Part III: comparisons of masticatory scores between two treatment modalities. J Prosthet Dent 65:272-283 [DOI] [PubMed] [Google Scholar]
- Lepley C, Throckmorton G, Parker S, Buschang PH. (2010). Masticatory performance and chewing cycle kinematics: are they related? Angle Orthod 80:295-301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedberg B, Norlen P, Owall B, Stoltze K. (2004). Masticatory and nutritional aspects on fixed and removable partial dentures. Clin Oral Investig 8:11-17 [DOI] [PubMed] [Google Scholar]
- Miyaura K, Morita M, Matsuka Y, Yamashita A, Watanabe T. (2000). Rehabilitation of biting abilities in patients with different types of dental prostheses. J Oral Rehabil 27:1073-1076 [DOI] [PubMed] [Google Scholar]
- Muller F, Hernandez M, Grütter L, Aracil-Kessler L, Weingart D, Schimmel M. (2012). Masseter muscle thickness, chewing efficiency and bite force in edentulous patients with fixed and removable implant-supported prostheses: a cross-sectional multicenter study. Clin Oral Implants Res 23:144-150 [DOI] [PubMed] [Google Scholar]
- Ohara Y, Hirano H, Watanabe Y, Edahiro A, Sato E, Shinkai S, et al. (2013). Masseter muscle tension and chewing ability in older persons. Geriatr Gerontol Int 13:372-377 [DOI] [PubMed] [Google Scholar]
- Pocztaruk Rde L, Frasca LC, Rivaldo EG, Fernandes Ede L, Gavião MB. (2008). Protocol for production of a chewable material for masticatory function tests (Optocal–Brazilian version). Braz Oral Res 22:305-310 [DOI] [PubMed] [Google Scholar]
- Raadsheer MC, van Eijden TM, van Ginkel FC, Prahl-Andersen B. (1999). Contribution of jaw muscle size and craniofacial morphology to human bite force magnitude. J Dent Res 78:31-42 [DOI] [PubMed] [Google Scholar]
- Trulsson M. (2006). Sensory-motor function of human periodontal mechanoreceptors. J Oral Rehabil 33:262-273 [DOI] [PubMed] [Google Scholar]
- Tsai CY, Lin YC, Su B, Yang LY, Chiu WC. (2012). Masseter muscle fibre changes following reduction of masticatory function. Int J Oral Maxillofac Surg 41:394-399 [DOI] [PubMed] [Google Scholar]
- van der Bilt A. (2011). Assessment of mastication with implications for oral rehabilitation: a review. J Oral Rehabil 38:754-780 [DOI] [PubMed] [Google Scholar]
- van der Bilt A, Fontijn-Tekamp FA. (2004). Comparison of single and multiple sieve methods for the determination of masticatory performance. Arch Oral Biol 49:193-198 [DOI] [PubMed] [Google Scholar]
- van der Bilt A, Engelen L, Pereira LJ, van der Glas HW, Abbink JH. (2006). Oral physiology and mastication. Physiol Behav 89:22-27 [DOI] [PubMed] [Google Scholar]
- van Kampen FM, van der Bilt A, Cune MS, Fontijn-Tekamp FA, Bosman F. (2004). Masticatory function with implant-supported overdentures. J Dent Res 83:708-711 [DOI] [PubMed] [Google Scholar]