Table 1.
Titration Experiments with 1 and Various Lewis Bases. 11B-NMR Data.a
| Lewis base | boronate complex (ppm) (2)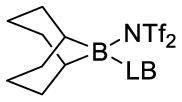
|
borenium ion (ppm) (3)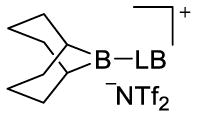
|
boronium ion (ppm) (4)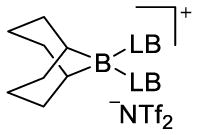
|
|---|---|---|---|
| Et3N (a) | 84.8 (85.1)b | ||
| DABCO (b) | 56.9 | ||
| pyridine (c) | 5.2 | ||
| 2,4-lutidine (d) | 39.4 | 9.7 | |
| 2,6-lutidine (e) | 84.4 | ||
| DMAP (f) | (66.5)b | (3)b | |
| proton sponge (g)c | (16.2)b | ||
| HMPA (h) | 64.9 | 17.8 | |
| Ph3P=O (i) | 67.4 | 17.3 | |
| n-Bu3P=O (j) | 65.1 | 14.5 | |
| Ph3P=S (k) | 80.4 | ||
| (Me2N)3P=S (l) | 80.9 | 46.9 | |
| pyridine-N-oxide (m) | 64.2 | 14.4 | |
| DMSO (n) | 66.1 | 15 | |
| n-Bu3P (o) | 33.1 | −5.4 | |
| benzophenone (p) | 35.6d |
11B NMR spectra were recorded at 128 MHz (CH2Cl2) with proton decoupling. Chemical shifts are reported in ppm from BF3•OEt2 (0.0 ppm) as the external standard.
Data in parentheses are from ref. 13.
1,8-bis(dimethylamino)naphthalene.
2.0 equiv of Lewis base.
