Table 2.
Hydrosilylation of Ketones Catalyzed by Various Boron Reagents.a
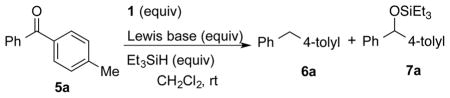
| ||||||
|---|---|---|---|---|---|---|
| entry | 1 (equiv) | Lewis base (equiv) | Et3SiH (equiv) | time | yieldb 6a (%) | yieldb 7a (%) |
| 1 | 0 | – | 1.5 | 6 h | 0 | 0 |
| 2 | 0.1 | – | 1.5 | 10 min | 69 | 0 |
| 3 | 0.1 | DABCO (0.1) | 1.0 | 12 h | 0 | 0 |
| 4 | 0.1 | 2,6-lutidine (0.1) | 1.5 | 30 min | <5 | 95 |
| 5 | 0.05 | 2,6-lutidine (0.05) | 1.5 | 20 h | <5 | 69 |
Reactions were performed at room temperature in an NMR tube (see Supporting Information for details).
Yields determined by integration of product signals versus Cl3CCH2Cl as an internal standard.
