Table 3.
Substrate Scope.a
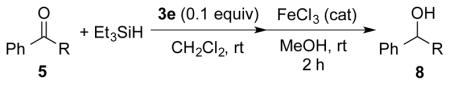
| |||||
|---|---|---|---|---|---|
| entry | R | ketone | time (min) | yieldb (%) | alcohol |
| 1 | 4-Me–C6H4 | 5a | 30 | 92 | 8a |
| 2 | 3-Me–C6H4 | 5b | 10 | 90 | 8b |
| 3 | 4-MeO–C6H4 | 5c | 180 | 91 | 8c |
| 4 | 4-Br–C6H4 | 5d | 10 | 93 | 8d |
| 5 | 1-naphthyl | 5e | 60 | 86 | 8e |
| 6 | 2-furyl | 5f | 10 | 82 | 8f |
| 7 | 2-thiophen | 5g | 60 | 83 | 8g |
| 8c | isopropyl | 5h | 60 | 77 | 8h |
Unless otherwise noted, reactions were performed on 0.1 mmol scale with 1.5 equiv of Et3SiH in dichloromethane (1.0 mL).
Yield of isolated, purified product.
0.2 equiv of 3e was used as catalyst.
