Abstract
The purpose of this study was to identify factors that are associated with experiencing genetic discrimination (GD) among individuals at risk for Huntington disease (HD). Multivariable logistic regression analysis was used to examine factors associated with experiencing GD in data from a cross-sectional, self-report survey of 293 individuals at risk for HD. The study sample comprised 167 genetically tested respondents, and 66 who were not tested (80% response rate). Overall, individuals who learn they are at risk for HD at a younger age (OR = 3.1; 95% CI: 1.5–6.2; P = 0.002), are mutation-positive (OR = 2.8; 95% CI: 1.4–6.0; P = 0.006), or are highly educated (OR = 2.7; 95% CI: 1.4–5.1; P = 0.002) are more likely to experience GD, particularly in insurance, family, and social settings. Further, younger age was associated with discrimination in insurance (OR = 0.97; 95% CI: 0.94–1.00; P = 0.038). This study provides evidence that some people who are at risk for HD were more likely to experience GD than others. Individuals who learned they are at risk for HD at a younger age and those who are mutation-positive were more likely to experience GD, particularly in insurance, family, and social settings. Younger individuals were more likely to experience discrimination in the insurance setting. Overall, highly educated individuals were also more likely to report discrimination. These results provide direction for clinical and family discussions, counseling practice, and policy aimed at mitigating experiences of GD.
Keywords: genetic discrimination, Huntington disease, genetic testing, socio-demographic factors
Introduction
The focus of genetic medicine has been shifting from genetic testing for monogenic disorders to genomic assessment of risk for common, complex disorders. Yet, despite substantial advances, uptake of genetic testing remains low. One reason is the fear of potential misuse and misunderstanding of genetic information in areas such as insurance and employment [Harper et al., 2004].
Genetic discrimination (GD) refers to differential treatment based on genetic differences as opposed to physical features [Billings et al., 1992]. Fear of GD has resulted in low participation rates in genetic testing programs and genetic research [Peterson et al., 2002; Hadley et al., 2003]. For example, many women in the US at increased risk for hereditary breast cancer decline testing for the BRCA1/2 susceptibility gene for fear of health insurance discrimination [Peterson et al., 2002]. Approximately half of them would have been found at high risk if they had been tested [Peterson et al., 2002], and thus deny themselves possible preventative management due to fear of discrimination.
US policymakers have responded to these concerns by implementing federal legislation aimed at prohibiting GD in health insurance and employment. Although the Genetic Information Non-discrimination Act of 2008 (GINA) was regarded as a breakthrough for genomic medicine, it does not require health insurers pay for interventions that genetic testing may indicate is necessary or prohibit use of genetic information in life, disability or long-term care insurance [Baruch and Hudson, 2008].
A recent study revealed that GD is frequent in these additional insurance contexts. Among 233 individuals at risk for Huntington disease (HD)—167 who had been tested for the HD mutation (83 tested positive and 84 tested negative), and 66 who had chosen not to have the genetic test—approximately 30% experienced discrimination with regard to life insurance or long-term disability insurance, or from mortgage companies [Bombard et al., 2009]. Discrimination was also reported in family (15.5%) and social (12.4%) situations, with fewer reports of employment (6.9%), health care (8.6%), or government-related (3.9%) discrimination. Further, having a family history of HD, even in the absence of a mutation-positive test result, is a major reason for experiences of GD [Bombard et al., 2009].
Alternative intervention strategies and support programs need to be developed to help individuals mitigate discrimination in these additional settings, and in countries where relevant protections do not exist. Although the nature and extent of GD in HD has been described, little is known about who is most at risk for experiencing discrimination, and thus for whom such strategies ought to be targeted [Pulst, 2009; Tibben, 2009]. The objective of this study was to describe the socio-demographic characteristics and factors that are associated with experiencing GD among individuals at risk for HD.
Methods
Study Design, Sample, and Measures
We conducted a cross-sectional survey at seven Canadian genetic and movement disorders clinics in 2006, targeting a total of 300 participants: those who were mutation-positive, mutation-negative, or not tested. We targeted individuals ≥18-year old who had a family history of HD and who were not symptomatic, which was defined as having a Unified Huntington Disease Rating Scale (UHDRS) score ≤2 and not having been diagnosed with signs of HD within the past year, by movement disorder specialists. We obtained ethics approval from relevant boards and informed consent from each respondent.
The survey design and administration, questionnaire content, and testing have been described in detail elsewhere [Bombard et al., 2009]. In brief, we selected and adapted questionnaire items on the basis of previous qualitative studies [Bombard et al., 2007, 2008; Penziner et al., 2008] and validated instruments [Krieger et al., 2005; Taylor et al., 2008]. The questionnaire was self-administered.
Respondents reported information about their age, sex, marital status, number of children, educational attainment, employment status, income, and ethnicity. To assess genetic test status, we asked “Have you had a genetic test for HD?” and “Did you get a positive test result (i.e., you have inherited the HD gene mutation)?” (Mutation status was also confirmed through medical records). We obtained information about when they learned of their genetic risk by asking: “In approximately what year did you first become aware that HD was in your family?” and “In what year did you have the genetic test?”
We assessed self-perceived experiences of discrimination by asking: “Have you ever experienced GD in any of the following situations because of your family history of HD?” and “Have you ever experienced GD in any of the following situations because of your genetic test result?” Each question was followed by a list of 23 possible situations of discrimination on the basis of either their family history or genetic test result (a list of 23 situations with the six subgroups are provided in Supplemental Table I). If participants responded “yes” to at least one situation, we classified them as having experienced discrimination. We described discrimination as being “unfairly prevented from doing something, or being treated unfairly,” based on the most common interpretation provided by participants in qualitative research that preceded survey development [Bombard et al., 2007, 2008; Penziner et al., 2008].
Statistical Analysis
We examined factors associated with experiences of GD in univariate logistic regression analyses, by setting (each with a binary outcome variable indicating whether the respondent had at least one perceived experience of GD in that setting or not). Due to the small sample sizes, settings were grouped as follows: insurance, family/social, and “other” (i.e., employment/health care/public sector). Then, we entered variables with P < 0.20 into a multivariable logistic regression model for each setting. We also estimated an overall model to illustrate factors associated with experiencing GD in the total sample, when all settings are combined.
The age when an individual learned of their family history is the difference between the age when they were surveyed and the number of years since they learned of their family history. We could not enter all three of these time-related variables in the same model because the third is completely determined by the other two, so we selected the two variables that were significant in the univariate analyses for inclusion in the multivariable model. The age of learning one’s family history was dichotomized at the median (age 25 years) in order to facilitate interpretation of the results (the results were similar whether it was entered as a continuous or categorical variable).
Two-sided P-values of 0.05 or less were considered to indicate statistical significance. We used SPSS 17.0.0 (SPSS Inc., Chicago, IL).
Results
Participants
We invited 299 individuals to participate in the survey, of whom 239 (79.9%) responded. We excluded six respondents who had UHDRS scores >2 after consenting to join the survey but before completing the survey questionnaire, yielding a sample size of 233, which represented a 79.5% response rate. Responders and non-responders did not differ significantly in terms of being tested or not (P = 0.334), or by positive or negative mutation status (P = 0.365), age (P = 0.077), or gender (P = 0.206). The socio-demographic characteristics of the respondents (Table I) were similar across all test status categories. The characteristics of our sample are similar to other HD study populations, although our sample is more educated and older [Wiggins et al., 1992; Almqvist et al., 2003]. The age when each respondent learned their family history of HD and the time interval (years) between learning the family history and completing the survey did not significantly differ across test status categories (Table II).
Table I.
Sociodemographic Characteristics of the Sample
| Total sample (n = 233) | Genetic test status categories | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Not tested (n = 66) | Mutation-negative (n = 84) | Mutation-positive (n = 83) | P-value | ||||||
|
| |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
| |||||||||
| Age (n = 233) | 45.5 | 11.7 | 47 | 11.0 | 46 | 13.7 | 42.9 | 9.5 | 0.085a |
|
| |||||||||
| n | % | n | % | n | % | n | % | ||
| a One-way ANOVA. | |||||||||
| b Pearson’s chi-square test (two-sided). | |||||||||
| c Does not meet assumptions of chi-squared test. | |||||||||
| d Self-reported. | |||||||||
| Sex (n = 233) | |||||||||
| Female | 153 | 65.7 | 48 | 72.7 | 52 | 61.9 | 53 | 63.9 | 0.349b |
| Marital status (n = 231) | |||||||||
| Single | 29 | 12.6 | 8 | 12.1 | 10 | 11.9 | 11 | 13.6 | —c |
| Married/common law | 176 | 76.2 | 53 | 80.3 | 59 | 70.2 | 64 | 79.0 | |
| Separated/divorced | 22 | 9.5 | 4 | 6.1 | 12 | 14.3 | 6 | 7.4 | |
| Widowed | 4 | 1.7 | 1 | 1.5 | 3 | 3.6 | 0 | 0.0 | |
| Children (n = 232) | |||||||||
| None | 63 | 27.2 | 22 | 33.3 | 20 | 23.8 | 21 | 25.6 | 0.518b |
| 1 | 36 | 15.5 | 8 | 12.1 | 12 | 14.3 | 16 | 19.5 | |
| >1 | 133 | 57.3 | 36 | 54.5 | 52 | 61.9 | 45 | 54.9 | |
| Education (n = 226) | |||||||||
| Below High school | 20 | 8.8 | 7 | 10.9 | 9 | 11.1 | 4 | 4.9 | 0.431b |
| Completed High school | 24 | 10.6 | 8 | 12.5 | 10 | 12.3 | 6 | 7.4 | |
| Completed University or teacher’s college | 73 | 32.3 | 16 | 25.0 | 28 | 34.6 | 29 | 35.8 | |
| Other post-secondary | 109 | 48.2 | 33 | 51.6 | 34 | 42.0 | 42 | 51.9 | |
| Personal income (n = 216) | |||||||||
| <$40,000 | 106 | 49.1 | 27 | 45.0 | 41 | 51.9 | 38 | 49.4 | 0.721b |
| $40,000 or more | 110 | 50.9 | 33 | 55.0 | 38 | 48.1 | 39 | 50.6 | |
| Employment (n = 228) | |||||||||
| Full-time | 140 | 61.4 | 46 | 71.9 | 46 | 56.1 | 48 | 58.5 | —c |
| Part-time | 25 | 11.0 | 8 | 12.5 | 9 | 11.0 | 8 | 9.8 | |
| Unemployed and seeking work | 11 | 4.8 | 4 | 6.3 | 3 | 3.7 | 4 | 4.9 | |
| Unemployed or retired and not seeking work | 52 | 22.8 | 6 | 9.4 | 24 | 29.3 | 22 | 26.8 | |
| Ethnicity (n = 230)d | |||||||||
| White or European | 227 | 98.7 | 64 | 98.5 | 82 | 98.8 | 81 | 98.8 | —c |
Table II.
Age When Learned of Family History and Years Since Learning of Family History of Huntington Disease
| Total sample | Genetic test status categories | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| n | % | Not tested | Mutation-negative | Mutation-positive | P-value | ||||
|
| |||||||||
| n | % | n | % | n | % | ||||
| a Pearson’s chi-square test (two-sided). | |||||||||
| Age when learned of family history (n = 211) | |||||||||
| <18 | 70 | 33.2 | 17 | 28.3 | 31 | 41.9 | 22 | 28.6 | 0.074a |
| 18–24 | 38 | 18.0 | 16 | 26.7 | 13 | 17.6 | 9 | 11.7 | |
| 25–39 | 58 | 27.5 | 18 | 30.0 | 16 | 21.6 | 24 | 31.2 | |
| 40+ | 45 | 21.3 | 9 | 15.0 | 14 | 18.9 | 22 | 28.6 | |
| Years since learned of family history (n = 211) | |||||||||
| <10 | 62 | 29.4 | 20 | 33.3 | 18 | 24.3 | 24 | 31.2 | 0.345a |
| 10–19 | 55 | 26.1 | 16 | 26.7 | 15 | 20.3 | 24 | 31.2 | |
| 20–29 | 48 | 22.7 | 14 | 23.3 | 19 | 25.7 | 15 | 19.5 | |
| 30+ | 46 | 21.8 | 10 | 16.7 | 22 | 29.7 | 14 | 18.2 | |
The prevalence of experiencing GD within each of the genetic test status categories in this sample has been described in detail elsewhere [Bombard et al., 2009]. As previously reported, 42 (50.6%) individuals who experienced discrimination were mutation-positive; 29 (34.5%) were mutation-negative; and, 22 (33.3%) had not been tested for HD [Bombard et al., 2009].
Factors Associated With Experiencing Genetic Discrimination
Based on the results of the univariate analyses (Table III), age, education level, age of learning of one’s family history of HD, and genetic test status were entered into multivariable models for each setting and for an “overall” model, which included all settings (Table IV).
Table III.
Univariate Analyses of Factors Associated With Self-Reported Genetic Discrimination, by Setting
| Insurance setting | Family/social settings | Other settingsa | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | P-valueb | OR | 95% CI | P-valueb | OR | 95% CI | P-valueb | |
| a “Other settings” include employment, health care, and government. | |||||||||
| b P-values <0.05 are highlighted in bold font. | |||||||||
| c Reference category. | |||||||||
| Age (n = 233) | |||||||||
| (Continuous variable) | 0.96 | 0.93–0.98 | 0.002 | 0.97 | 0.94–1.00 | 0.048 | 0.98 | 0.95–1.01 | 0.160 |
| Sex (n = 233) | |||||||||
| Female | 0.72 | 0.40–1.29 | 0.269 | 1.42 | 0.70–2.88 | 0.334 | 1.11 | 0.51–2.41 | 0.792 |
| Malec | |||||||||
| Children (n = 232) | |||||||||
| Have children | 0.95 | 0.50–1.78 | 0.862 | 1.07 | 0.51–2.23 | 0.856 | 1.04 | 0.46–2.37 | 0.923 |
| Do not have childrenc | |||||||||
| Marital status (n = 231) | |||||||||
| Married | 1.47 | 0.73–2.95 | 0.281 | 0.99 | 0.47–2.12 | 0.985 | 1.24 | 0.51–3.03 | 0.633 |
| Not marriedc | |||||||||
| Highest level of education (n = 226) | |||||||||
| Completed university or teachers college | 2.07 | 1.14–3.75 | 0.017 | 1.72 | 0.88–3.36 | 0.114 | 0.85 | 0.38–1.89 | 0.696 |
| Have not completed university or teachers collegec | |||||||||
| Personal income (n = 216) | |||||||||
| $40,000 or more | 1.14 | 0.63–2.08 | 0.661 | 1.07 | 0.55–2.08 | 0.841 | 1.11 | 0.52–2.35 | 0.788 |
| <$40,000c | |||||||||
| Employment (n = 228) | |||||||||
| Currently employed | 1.43 | 0.73–2.79 | 0.292 | 1.27 | 0.60–2.69 | 0.529 | 0.90 | 0.40–2.01 | 0.801 |
| Not currently employedc | |||||||||
| Age when learned of family history of HD (n = 211) | |||||||||
| Under age 25 | 0.40 | 0.22–0.73 | 0.003 | 0.36 | 0.18–0.73 | 0.005 | 0.47 | 0.21–1.02 | 0.056 |
| 25 years old or olderc | |||||||||
| Years since learned of family history of HD (n = 211) | |||||||||
| (Continuous variable) | 1.01 | 0.99–1.04 | 0.276 | 1.02 | 0.99–1.04 | 0.230 | 1.01 | 0.98–1.04 | 0.579 |
| Genetic test status (n = 233) | |||||||||
| Have not been tested for HD | 1.21 | 0.57–2.56 | 0.625 | 1.45 | 0.57–3.70 | 0.436 | 1.01 | 0.36–2.88 | 0.983 |
| Mutation-positive | 2.03 | 0.98–4.20 | 0.057 | 2.95 | 1.23–7.10 | 0.016 | 2.33 | 0.91–5.98 | 0.078 |
| Mutation-negativec | |||||||||
Table IV.
Multivariable Analyses of Factors Associated With Self-Reported Genetic Discrimination, by Setting and Overall
| Insurance setting | Family/social settings | Other settingsa | Overallb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| OR | 95% CI | P-valuec | OR | 95% CI | P-valuec | OR | 95% CI | P-valuec | OR | 95% CI | P-valuec | |
| a The “Other settings” category includes employment, health care, and government. | ||||||||||||
| b The “Overall” category includes all settings. | ||||||||||||
| c P-values <0.05 are highlighted in bold font. | ||||||||||||
| d Reference category. | ||||||||||||
| Age (n = 233) | ||||||||||||
| (Continuous variable) | 0.97 | 0.94–1.00 | 0.038 | 0.99 | 0.96–1.03 | 0.705 | 0.98 | 0.94–1.02 | 0.258 | 0.98 | 0.95–1.01 | 0.172 |
| Highest level of education (n = 226) | ||||||||||||
| Completed university or teachers college | 1.66 | 0.86–3.17 | 0.129 | 1.43 | 0.68–3.00 | 0.351 | 0.79 | 0.34–1.80 | 0.571 | 2.71 | 1.43–5.13 | 0.002 |
| Have not completed university or teachers colleged | ||||||||||||
| Age when learned of family history of HD (n = 211) | ||||||||||||
| Under age 25 | 2.35 | 1.13–4.86 | 0.022 | 4.08 | 1.68–9.93 | 0.002 | 2.03 | 0.82–5.01 | 0.124 | 3.08 | 1.53–6.20 | 0.002 |
| 25 years old or olderd | ||||||||||||
| Genetic test status (n = 233) | ||||||||||||
| Have not been tested for HD | 0.80 | 0.34–1.84 | 0.592 | 0.95 | 0.34–2.65 | 0.925 | 0.91 | 0.31–2.68 | 0.869 | 1.07 | 0.49–2.37 | 0.860 |
| Mutation-positive | 2.26 | 1.06–4.82 | 0.036 | 3.83 | 1.59–9.21 | 0.003 | 2.43 | 0.97–6.10 | 0.058 | 2.84 | 1.35–6.00 | 0.006 |
| Mutation-negatived | ||||||||||||
In the insurance setting, age, mutation status, and the age of learning of one’s family history of HD were significantly associated with GD. Individuals who learned of their family history at an earlier age (OR = 2.35; 95% CI: 1.13–4.86; P = 0.022) and younger respondents (OR = 0.97; 95% CI: 0.94–1.00; P = 0.038) were more likely to experience GD in the insurance setting. In addition, those who were mutation-positive were more likely to experience GD than those who were mutation-negative (OR = 2.26; 95% CI: 1.06–4.82; P = 0.036).
In the family/social setting, age of learning of one’s family history of HD and mutation-positive status were significantly associated with GD. Individuals who discovered that they are at risk for HD at a younger age (OR = 4.08; 95% CI: 1.68–9.93; P = 0.002) and those who are mutation-positive (OR = 3.83; 95% CI: 1.59–9.21; P = 0.003) were more likely to experience discrimination in social/family settings.
In the other settings (i.e., employment, health care, and public sector), no statistically significant associations were observed.
In the “overall” model that combined all settings, education (OR = 2.71; 95% CI: 1.43–5.13; P = 0.002), the age of learning of one’s family history of HD (OR = 3.08; 95% CI: 1.53–6.20; P = 0.002), and mutation-positive status (OR = 2.84; 95% CI: 1.35–6.00; P =0.006), were significantly associated with GD, but age was not significant.
There was no significant difference between being mutation-negative versus not being tested in any of the settings or overall model.
Discussion
Our study provides novel insights into the factors associated with GD. Individuals who learned they are at risk at a younger age and those who are mutation-positive were more likely to experience GD, particularly in insurance, family, and social settings. Highly educated individuals were also more likely to report discrimination, when all settings are combined. Further, younger individuals were more likely to experience discrimination in the insurance setting. These results offer insights for clinicians supporting individuals through genetic testing and counseling to determine whom among the at-risk population is most likely to experience GD.
The age when one discovers the family history of HD appears to be a salient factor associated with GD, particularly in the insurance, and family/social settings. This finding is consistent with previous research, which has shown that the family history is the major reason for GD [Bombard et al., 2009] and that children’s age is a critical factor in parents’ decisions about when to inform them of their genetic risk [Forrest et al., 2003; Gaff et al., 2007; Klitzman et al., 2007; Forrest Keenan et al., 2009]. Communicating genetic risk to children is a complex issue for families; and, for parents of HD families, in particular [Forrest et al., 2003]. It is not clear what the optimal age is when young people should be informed about their genetic risk for HD. Some studies suggest that adult children prefer early disclosure of genetic risk; and, that growing up knowing about HD at an early age allows youth to cope better when relatives are symptomatic and removes the veil of secrecy around HD in families [van der Steenstraten et al., 1994; Forrest et al., 2003; Klitzman et al., 2007; Forrest Keenan et al., 2009]. However, other studies point to children’s preferences to be told later [Klitzman et al., 2007; Forrest Keenan et al., 2009]. Often relatives “decline to tell” their families about their genetic risk [Forrest et al., 2003] as a way to protect them as long as possible from its consequences, including the potential of GD [Forrest et al., 2003; Klitzman et al., 2007]. Ultimately, choosing the appropriate time to inform relatives of their family history involves a delicate balance where the potential of harm (or benefit) of imparting this news must be weighed against the responsibility to share important information that may shape future decisions [Gaff et al., 2007].
One could hypothesize that the younger a person is when they learn about their family history, the more likely that information becomes relevant in subsequent life events, including insurance contracts, career decisions, family- and general life-planning. In fact, studies support this hypothesis, indicating that a family history of HD is a crucial framework for personal decision-making regarding reproductive, marital, career, and predictive testing choices [Cox, 1999; Taylor, 2004], and an individual’s stage of life is an important factor in his/her engagement with GD [Bombard et al., 2008]. Arguably, learning about a genetic risk later in life—after insurance arrangements, career, and reproductive decisions are set in place—may reduce the likelihood of GD. Indeed, in our study those who learned of their family history after the age of 25 were less likely to experience GD (Fig. 1). This relationship persisted when we adjusted for the age of the respondents when they were surveyed. One explanation is that individuals who became aware of their family history earlier simply had more time to encounter episodes of discrimination.
Figure 1.
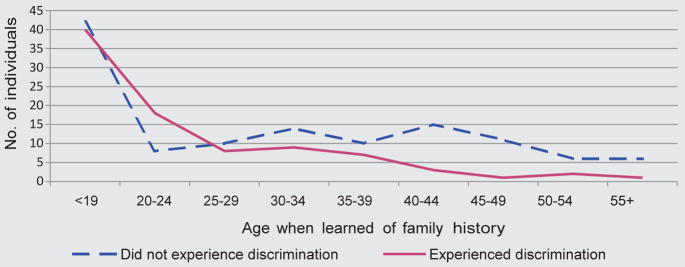
Number of respondents who experienced discrimination by the age when they learnend of their family history of HD.
However, this relationship was not dependent on the number of years since the respondents learned their family history. It is possible that those who learned their family history early had a parent whose diagnosis occurred at an earlier age and the reported discrimination was because they were living with an affected parent.
Despite our results that link the early discovery of the family history of HD with experiencing GD, there are many factors that come into play when considering such disclosure, some of which include: families’ style of communication [Forrest et al., 2003; Gaff et al., 2007], the nature of the disease [Wilson et al., 2004], and the availability of treatment [Wilson et al., 2004]. Arguably, the increasing discovery of presymptomatic biomarkers for HD [Paulsen et al., 2008; Tabrizi et al., 2009] may facilitate the early initiation of treatment so that the benefit of protective treatment would outweigh the potential risks in the early discovery of a family history of HD. Clearly then, the potential for discrimination represents only one piece of a larger puzzle families must grapple with when timing the disclosure of the family history of HD to relatives.
Receiving a mutation-positive test result confers near-certain risk of disease. Overall, mutation-positive individuals were nearly three times more likely to experience GD than mutation-negative respondents. However, these findings do not imply that mutation-negative individuals do not experience GD. Based on our analyses, there was a similar likelihood of experiencing GD among mutation-negative individuals as those who had not been tested in all settings. Indeed, previous research based on this sample showed that 34.5% of the 84 mutation-negative respondents, as well as 33.3% of the 66 respondents who had not been tested for HD, experienced GD, primarily due to their family history [Bombard et al., 2009]. Taken together, these findings underscore the fact that learning about a family history early confers risk of GD, and mutation-positive status may make discrimination even more likely.
Overall, education was significantly associated with GD. Respondents who experienced discrimination were almost three times more likely to have completed university or teachers college. Higher educational attainment has also been associated with reports of racial [Borrell et al., 2007] and gender [Watson et al., 2002] discrimination. While university completion is indicative of higher socioeconomic status, we did not find a significant association between experiencing discrimination and either the respondents’ income or employment status. An alternative explanation for this relationship could be that education may be linked to a general awareness of contemporary issues, one of which is GD. This awareness may influence how individuals perceive, or engage with GD [Bombard et al., 2008]. Individuals highly engaged with GD may more readily identify unfair treatment as discriminatory as opposed to minimizing or dismissing its occurrence [Bombard et al., 2007]. Moreover, individuals with higher education levels may be more informed about genetic issues. For example, in a post hoc analysis, the proportion of individuals who completed university who rated their knowledge of the possible advantages and disadvantages of genetic testing as 4 or more (on a scale of 1–5) was significantly greater than the proportion of those with lower education levels (82.9% vs. 66.7%; P = 0.013).
Although education was significantly associated with GD when all of the settings were combined (OR = 2.7; P = 0.002), this relationship did not achieve statistical significance in the multivariable models by setting. We observed that the odds ratios for the association between high education levels and GD in the insurance and family/social settings were in the same direction (OR > 1) as the overall relationship. An exception occurred in the other settings (employment, health care, and public sector), where an inverse relationship (OR < 1) occurred. Further research with larger samples to elucidate the nature of the relationship is warranted.
Younger individuals were more likely to report experiences of discrimination when applying for insurance. This finding may indicate that insurers are currently requesting family history and genetic test results as part of their applications. This is consistent with results from an informal survey of Canadian life insurance contracts from 2004, where over 90% of insurance companies ask about the presence of a genetic disease in the family, and specifically HD in many cases [Harper et al., 2004]. With the proliferation of genetic testing, implicit or explicit questions regarding genetic information in insurance applications are likely to increase and our finding that younger respondents were more likely to report discrimination in insurance appears consistent with this explanation.
The introduction of GINA was a significant step forward for individuals concerned with participating in genetic testing or research in the US. However, with its focus on health insurance and employment, GINA does not address commonly occurring life and disability insurance discrimination, family and social discrimination, and the potential for unfair treatment in health care, government, education, and other settings [Bombard et al., 2009]. Moreover, few other G8 countries have legislative protections in place, and where these exist they are similarly limited to prohibiting discrimination in insurance and employment sectors (Table V). Therefore, other approaches are also needed.
Table V.
Regulatory Structures Concerning the Use of Genetic Information by Insurers and Employers Across G8 Countries
| Country | Insurance
|
Employment Legislation | |
|---|---|---|---|
| Moratorium | Legislation | ||
| Canada | Partiala | No | No |
| France | Yes | Yes | Yesc |
| Germany | Yes | Yes | Yesd |
| Italy | No | No | Yese |
| Japan | No | No | No |
| Russia | No | No | No |
| United Kingdom | Limitedb | No | No |
| United States | No | Yes | Yesf |
Partial moratorium: insurers would not request that an applicant undergo testing, but may request the results of previous tests.
Limited moratorium: insurers would not ask an applicant to undergo genetic testing or request previous test results for policies under a certain dollar amount. Beyond this “threshold” or “ceiling”, insurers would be permitted to request and use the genetic testing in their premium assessments.
Legislation prohibits genetic testing by employers.
Legislation allows the use of genetic tests for life, disability, occupational, and pension insurance contracts exceeding EUR 300,000.
Legislation allows employers to use genetic tests where there is an unambiguous health requirement for the job.
Legislation prohibits use of genetic information in job-related decisions.
Adapted, with permission, from Lemmens et al. [2004] Genetics and Life Insurance: A comparative analysis. GenEdit
Initiating public education campaigns about genetics would increase the public’s awareness and may reduce stigma. Such initiatives have been undertaken in Australia and the UK, where genetic education centers promote public awareness and professional education on genetics and provide educational materials on the potential of GD [New South Wales Health, 2007; North West Genetics Knowledge Parks, 2009]. Most clinicians are unaware of GD laws in their jurisdictions [Nedelcu et al., 2004], and this lack of information may influence their decision to refer patients for genetic testing [Nedelcu et al., 2004], effectively creating a barrier to those who may benefit from possible psychological relief, preventative management, or treatment opportunities.
Health providers are in a unique position to help testing candidates mitigate GD. Given the reported occurrence of discrimination in family settings [Bombard et al., 2009], it is important for providers to discuss patients’ plans for disclosure of test results during pretest counseling. Raising the potential reactions within families and with friends may provide an opportunity to tailor strategies to cope with such consequences. Other potential strategies have been previously identified to manage GD in insurance and employment contexts [Bombard et al., 2007], and include: anonymous testing [Burgess et al., 1997], maintaining shadow charts [Bower et al., 2002], and not sending a letter to family physicians so that they, along with insurers and employers, remain unaware of an individual’s involvement in predictive testing. These strategies raise salient issues with regard to optimal care and information sharing, and thus ought to be used cautiously.
Our findings should be considered in the context of several limitations. The results provide general factors associated with experiencing GD and did not take into account the timing of the experience with respect to either learning the family history or the individual’s current age, or the number of affected relatives in a pedigree. We also relied on self-reported data, and it is possible that respondents may not have accurately recalled when they learned their family history. Some individuals learn of their family history at a discrete point in time, while others acquire this awareness gradually [Cox, 1999]. Although we relied on the survey respondents’ reported experiences of discrimination rather than confirmed episodes, cases of consumer-reported experiences of GD have been verified by others [Barlow-Stewart et al., 2009]. Nevertheless, it is possible that some people are more likely than others to feel that they have suffered GD in similar circumstances.
Our sample was recruited from participants in predictive testing and genetic research, who may differ from other individuals who are at risk of HD. Our sample had higher education levels than samples in other studies of HD populations [Wiggins et al., 1992; Almqvist et al., 2003]. Until further research is conducted on a broader sample, our results should be generalized cautiously. Our study was based in Canada, and the findings may not be generalizable to other countries or to populations who do not have access to universal publicly funded health care.
Genetic discrimination was rare in certain settings in this sample: few individuals experienced discrimination in the employment (n = 16), health care (n = 20), or public sector settings (n = 9). Even though we combined these settings to create a large sample size for the analyses (n = 34), there may have been too few observations to detect statistically significant associations. Moreover, although these settings were conceptually similar, we have presented the combined results with the caveat that there may have been differences among these three settings that affected the precision of the estimates. We performed multiple analyses, which can increase the probability of obtaining a significant result. However, this would not likely explain the findings in this study because the results were similar across the models, and the P-values in the overall model were quite small.
In conclusion, this study provides evidence that some people who are at risk for HD were more likely to experience GD than others. These results provide direction for clinical and family discussions, counseling practice, and policy aimed at mitigating experiences of GD.
Supplementary Material
Acknowledgments
We are indebted to the families who made this study possible by sharing their experiences with us. We also wish to thank Lauren Currie for support in the collection and entry of the data. Funding for this study from the Canadian Institutes of Health Research (CIHR) was awarded to Michael Hayden and Joan Bottorff. Supplemental funding from the National Institutes of Health, National Institute of Neurological Disorders and Stroke was awarded to Jane Paulsen (number 3 R01 NS040068). Yvonne Bombard was supported by the CIHR and the Michael Smith Foundation for Health Research/Child and Family Research Institute; and is currently funded by the CIHR Strategic Training Fellowships of “Public Health Policy” and “Health Care, Technology and Place.”
References
- Almqvist EW, Brinkman RR, Wiggins S, Hayden MR. Psychological consequences and predictors of adverse events in the first 5 years after predictive testing for Huntington’s disease. Clin Genet. 2003;64(4):300–309. doi: 10.1034/j.1399-0004.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- Barlow-Stewart K, Taylor SD, Treloar SA, Stranger M, Otlowski M. Verification of consumers’ experiences and perceptions of genetic discrimination and its impact on utilization of genetic testing. Genet Med. 2009;11(3):193–201. doi: 10.1097/GIM.0b013e318194ee75. [DOI] [PubMed] [Google Scholar]
- Baruch S, Hudson K. Civilian and military genetics: Nondiscrimination policy in a post-GINA world. Am J Hum Genet. 2008;83(4):435–444. doi: 10.1016/j.ajhg.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billings PR, Kohn MA, de Cuevas M, Beckwith J, Alper JS, Natowicz MR. Discrimination as a consequence of genetic testing. Am J Hum Genet. 1992;50(3):476–482. [PMC free article] [PubMed] [Google Scholar]
- Bombard Y, Penziner E, Decolongon J, Klimek ML, Creighton S, Suchowersky O, Guttman M, Paulsen JS, Bottorff JL, Hayden MR. Managing genetic discrimination: Strategies used by individuals found to have the Huntington disease mutation. Clin Genet. 2007;71(3):220–231. doi: 10.1111/j.1399-0004.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- Bombard Y, Penziner E, Suchowersky O, Guttman M, Paulsen JS, Bottorff JL, Hayden MR. Engagement with genetic discrimination: Concerns and experiences in the context of Huntington disease. Eur J Hum Genet. 2008;16(3):279–289. doi: 10.1038/sj.ejhg.5201937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombard Y, Veenstra G, Friedman JM, Creighton S, Currie L, Paulsen JS, Bottorff JL, Hayden MR. Perceptions of genetic discrimination among people at risk for Huntington’s disease: A cross sectional survey. BMJ. 2009;338:b2175. doi: 10.1136/bmj.b2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrell LN, Jacobs DR, Jr, Williams DR, Pletcher MJ, Houston TK, Kiefe CI. Self-reported racial discrimination and substance use in the Coronary Artery Risk Development in Adults Study. Am J Epidemiol. 2007;166(9):1068–1079. doi: 10.1093/aje/kwm180. [DOI] [PubMed] [Google Scholar]
- Bower MA, Veach PM, Bartels DM, LeRoy BS. A survey of genetic counselors’ strategies for addressing ethical and professional challenges in practice. J Genet Couns. 2002;11(3):163–186. doi: 10.1023/a:1015275022199. [DOI] [PubMed] [Google Scholar]
- Burgess MM, Adam S, Bloch M, Hayden MR. Dilemmas of anonymous predictive testing for Huntington disease: Privacy vs. optimal care. Am J Med Genet. 1997;71(2):197–201. [PubMed] [Google Scholar]
- Cox SM, McKellin W. ‘There’s this thing in our family’: Predictive testing and the construction of risk for Huntington Disease. Sociol Health Illn. 1999;21:622–646. [Google Scholar]
- Forrest K, Simpson SA, Wilson BJ, van Teijlingen ER, McKee L, Haites N, Matthews E. To tell or not to tell: barriers and facilitators in family communication about genetic risk. Clin Genet. 2003;64(4):317–326. doi: 10.1034/j.1399-0004.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- Forrest Keenan K, van Teijlingen E, McKee L, Miedzybrodzka Z, Simpson SA. How young people find out about their family history of Huntington’s disease. SocSci Med. 2009;68(10):1892–1900. doi: 10.1016/j.socscimed.2009.02.049. [DOI] [PubMed] [Google Scholar]
- Gaff CL, Clarke AJ, Atkinson P, Sivell S, Elwyn G, Iredale R, Thornton H, Dundon J, Shaw C, Edwards A. Process and outcome in communication of genetic information within families: A systematic review. Eur J Hum Genet. 2007;15(10):999–1011. doi: 10.1038/sj.ejhg.5201883. [DOI] [PubMed] [Google Scholar]
- Hadley DW, Jenkins J, Dimond E, Nakahara K, Grogan L, Liewehr DJ, Steinberg SM, Kirsch I. Genetic counseling and testing in families with hereditary nonpolyposis colorectal cancer. Arch Intern Med. 2003;163(5):573–582. doi: 10.1001/archinte.163.5.573. [DOI] [PubMed] [Google Scholar]
- Harper PS, Gevers S, de Wert G, Creighton S, Bombard Y, Hayden MR. Genetic testing and Huntington’s disease: Issues of employment. Lancet Neurol. 2004;3(4):249–252. doi: 10.1016/S1474-4422(04)00711-2. [DOI] [PubMed] [Google Scholar]
- Klitzman R, Thorne D, Williamson J, Chung W, Marder K. Disclosures of Huntington disease risk within families: Patterns of decision-making and implications. Am J Med Genet Part A. 2007;143A(16):1835–1849. doi: 10.1002/ajmg.a.31864. [DOI] [PubMed] [Google Scholar]
- Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: Validity and reliability of a self-report measure for population health research on racism and health. SocSci Med. 2005;61(7):1576–1596. doi: 10.1016/j.socscimed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Nedelcu R, Blazer KR, Schwerin BU, Gambol P, Mantha P, Uman GC, Weitzel JN. Genetic discrimination: The clinician perspective. Clin Genet. 2004;66(4):311–317. doi: 10.1111/j.1399-0004.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- New South Wales Health. Centre for Genetics Education. 2007. [Google Scholar]
- North West Genetics Knowledge Parks. Nowgen—A Centre for Genetics in Healthcare. 2009. [Google Scholar]
- Paulsen JS, Langbehn DR, Stout JC, Aylward E, Ross CA, Nance M, Guttman M, Johnson S, MacDonald M, Beglinger LJ, et al. Detection of Huntington’s disease decades before diagnosis: The Predict-HD study. J Neurol Neurosurg Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penziner E, Williams JK, Erwin C, Bombard Y, Wallis A, Beglinger LJ, Hayden MR, Paulsen JS. Perceptions of discrimination among persons who have undergone predictive testing for Huntington’s disease. Am J Med Genet Part B. 2008;147B(3):320–325. doi: 10.1002/ajmg.b.30600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EA, Milliron KJ, Lewis KE, Goold SD, Merajver SD. Health insurance and discrimination concerns and BRCA1/2 testing in a clinic population. Cancer Epidemiol Biomarkers Prev. 2002;11(1):79–87. [PubMed] [Google Scholar]
- Pulst SM. Neurodegenerative disease. Genetic discrimination in Huntington disease. Nat Rev Neurol. 2009;5(10):525–526. doi: 10.1038/nrneurol.2009.153. [DOI] [PubMed] [Google Scholar]
- Tabrizi SJ, Langbehn DR, Leavitt BR, Roos RA, Durr A, Craufurd D, Kennard C, Hicks SL, Fox NC, Scahill RI, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: Cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SD. Predictive genetic test decisions for Huntington’s disease: Context, appraisal and new moral imperatives. SocSci Med. 2004;58(1):137–149. doi: 10.1016/s0277-9536(03)00155-2. [DOI] [PubMed] [Google Scholar]
- Taylor S, Treloar S, Barlow-Stewart K, Stranger M, Otlowski M. Investigating genetic discrimination in Australia: A large-scale survey of clinical genetics clients. Clin Genet. 2008;74(1):20–30. doi: 10.1111/j.1399-0004.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Tibben A. Genetic discrimination in Huntington’s disease. BMJ. 2009;338:b1281. doi: 10.1136/bmj.b1281. [DOI] [PubMed] [Google Scholar]
- van der Steenstraten IM, Tibben A, Roos RA, van de Kamp JJ, Niermeijer MF. Predictive testing for Huntington disease: Nonparticipants compared with participants in the Dutch program. Am J Hum Genet. 1994;55(4):618–625. [PMC free article] [PubMed] [Google Scholar]
- Watson JM, Scarinci IC, Klesges RC, Slawson D, Beech BM. Race, socioeconomic status, and perceived discrimination among healthy women. J Womens Health Gend Based Med. 2002;11(5):441–451. doi: 10.1089/15246090260137617. [DOI] [PubMed] [Google Scholar]
- Wiggins S, Whyte P, Huggins M, Adam S, Theilmann J, Bloch M, Sheps SB, Schechter MT, Hayden MR. The psychological consequences of predictive testing for Huntington’s disease. Canadian Collaborative Study of Predictive Testing. N Engl J Med. 1992;327(20):1401–1405. doi: 10.1056/NEJM199211123272001. [DOI] [PubMed] [Google Scholar]
- Wilson BJ, Forrest K, van Teijlingen ER, McKee L, Haites N, Matthews E, Simpson SA. Family communication about genetic risk: The little that is known. Community Genet. 2004;7(1):15–24. doi: 10.1159/000080300. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


