Fig. 3.
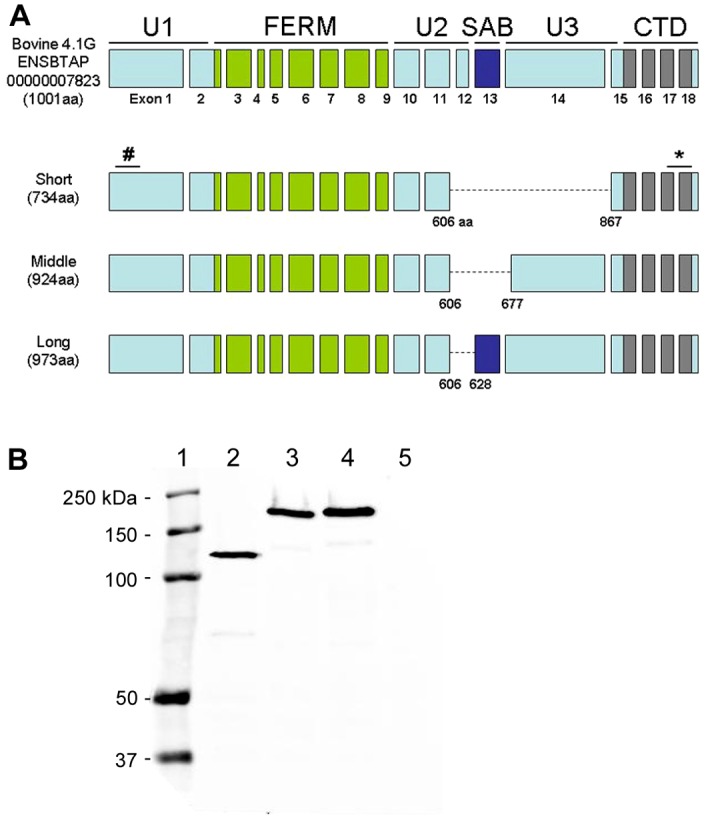
Characterization of retinal 4.1G. (A) Schematic representation of domain and exon structures of the predicted 4.1G protein in comparison with the cloned retinal 4.1G variants. The epitope of the monoclonal antibody against bovine 4.1G was mapped to amino acid residues 62–112 (#). Anti-4.1G polyclonal antibody was generated against a peptide containing amino acid residues 965–984 (*). (B) Western blot of HEK-293 cell lysates labeled with Rho-1D4 antibody. HEK-293 cells were transfected with bovine 4.1G short (lane 2), middle (lane 3) and long (lane 4) variants each having a 1D4 tag at the C-terminus. Non-transfected HEK-293 cell lysate was loaded as control (lane 5). Molecular markers were loaded as reference (lane 1).
