Fig. 5.
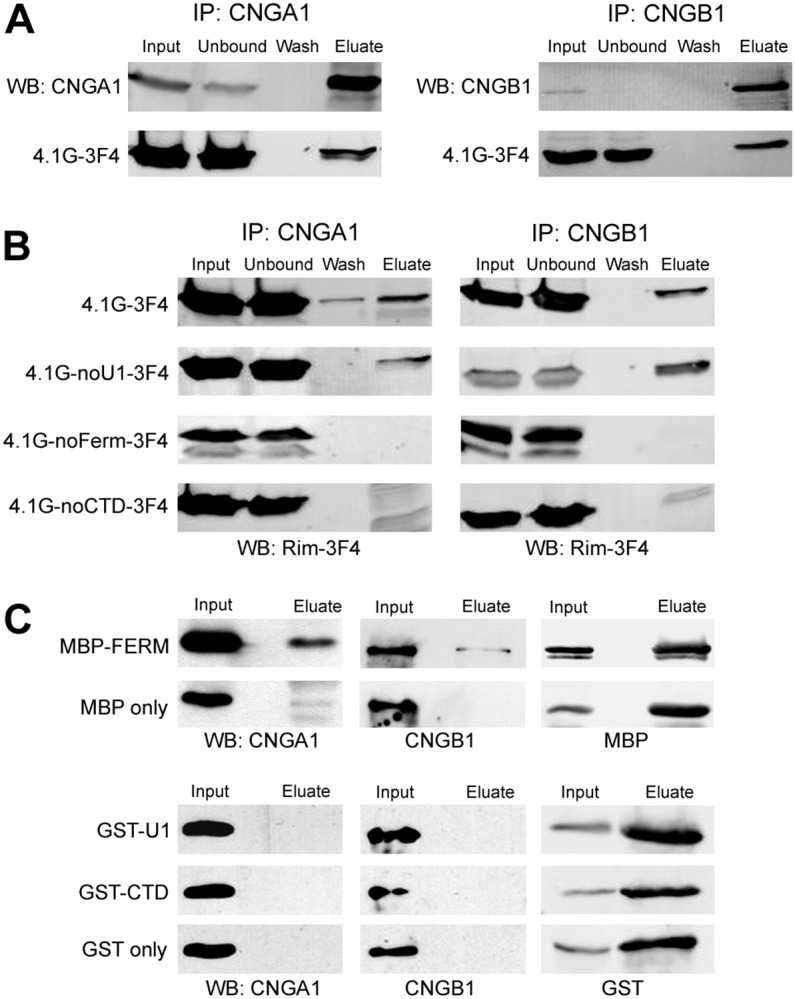
Domains of 4.1G required for binding to CNG channels. (A) Co-immunoprecipitation of 4.1G with CNG channels. Lysates of HEK-293 cells co-transfected with 4.1G-3F4 and CNGA1 (left panel) or CNGB1 (right panel) were incubated with anti-CNGA1 (PMc-1D1) or anti-CNGB1 (Garp-8G8) antibody column, respectively. Western blots of various fractions (input, unbound, wash and eluate) were labeled with anti-CNGA1 (top left) or anti-CNGB1 (top right) antibody and Rim-3F4 antibody (bottom). (B) Co-immunoprecipitation of 4.1G deletion mutants with CNG channels. Lysates of HEK-293 cells co-transfected with deletion construct of 4.1G and CNGA1 (left panel) or CNGB1 (right panel) were incubated with anti-CNGA1 or anti-CNGB1 antibody column, respectively. Western blots were labeled with Rim-3F4 to detect 4.1G full length (top) or deletion mutants (bottom 3) all of which had a 3F4 tag at the C-terminus. Note that 4.1G lacking the FERM domain or the CTD domain failed to co-immunoprecipitate with CNGA1 and CNGB1. (C) Co-immunoprecipitation of CNG channels with MBP–FERM. The domains of 4.1G (U1, FERM, CTD) were fused to either MBP or GST and incubated with bovine ROS and immunoprecipitated with either amylose resin or glutathione resin. Western blots of the input and eluate fractions were labeled with anti-CNGA1 (left panel), anti-CNGB1 antibody (middle panel) or an antibody against MBP or GST (right panel). Note that CNGA1 and CNGB1 were co-immunoprecipitated with MBP–FERM only. MBP and GST alone were used as control.
