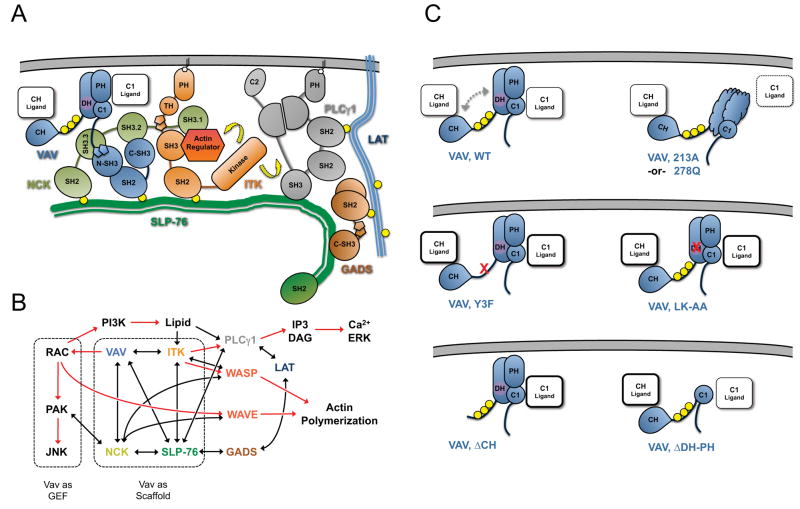
Figure 7. Model of Vav1-mediated scaffolding interactions within SLP-76 microclusters.
(A) Interactions within the signaling complex nucleated on the N-terminal tyrosines of SLP-76. Vav1, Nck, and Itk are recruited to the phosphorylated tyrosines (yellow circles) of SLP-76 where they participate in multivalent scaffolding interactions. The interactions conformationally activate and optimally position Itk, enabling it to activate (yellow arrows) known substrates, such as PLC γ1 and the actin regulator WASP. (B) Involvement of Vav1 in signaling pathways leading to T cell activation. Known biochemical interactions are represented by black lines and postulated interactions by dotted lines. The activation of downstream effectors is indicated by red arrows. Vav1 GEF-dependent and GEF-independent pathways are highlighted with dashed boxes. (C) The predicted conformation of and scaffolding interactions mediated by the N-terminus of Vav1 under diverse conditions, based on the crystal structure of the auto-inhibited N-terminus and the functional and imaging data presented here. In wild-type Vav1, phosphorylation (yellow circles) relieves the autoinhibitory interaction of the CH with the GEF cassette (dashed arrows), permitting ligand binding by the CH, PH and C1 domains. The L213A and L278Q mutations are predicted to disrupt domain folding and to occlude a novel binding surface on the C1 domain. The remaining mutations are expected to perturb the auto-inhibitory fold and may increase binding of ligands to the remaining domains.