Highlights
► 33 years old woman diagnosed with choriocarcinoma after delivery, her newborn son with the same diagnosis. ► Case of transplasental dissemination of choriacarcinoma. ► Advanced disease both in the mother and the infant.
Keywords: Infantile choriocarcinoma, Choriocarcinoma
Introduction
Choriocarcinoma is a rare malignant tumor of the syncytiotrophoblast and cytotrophoblast that may occur after any pregnancy. Approximately one half of choriocarcinomas occur following a hydatidaform mole and the remainder after other pregnancies, including normal ones (Loukovaara et al., 2005). The incidence after a normal delivery is estimated to be 1 in 50,000 live births (Tidy et al., 1995). Although the malignancy originates in the placenta, there is often widespread metastasis, most often to the lungs, vagina, pelvis, brain, and liver (Berkowitz and Goldstein, 1981).
While maternal choriocarcinoma is quite uncommon, simultaneous malignancy in both mother and infant occurs with far less frequency. Infantile choriocarcinoma is thought to be of the same origins as of maternal disease, and is prone to metastasis as well, usually to the liver. Affected newborns often present with anemia, hepatomegaly, and precocious puberty (van der Hoef et al., 2004).
Despite the aggressive nature of the tumor, it is extremely sensitive to chemotherapeutic agents, making it a highly curable malignancy. Because the syncytiotrophoblast and cytotrophoblast both secrete β-hCG, plasma levels of the hormone are typically elevated with disease and are used to follow, the response to treatment (Blohm et al., 2001).
The following details the presentation and management of a case of simultaneous maternal and infantile choriocarcinoma.
Case report
A 33 year old gravida 1 para 0 pregnant woman had a relatively uncomplicated pregnancy with the exception of cramping at 12 weeks, hemoptysis at 28 weeks, and rib pain at 36 weeks. Computed tomography (CT) scan at 36 weeks showed bilateral numerous pulmonary nodules measuring up to 3.5 cm as well as a 4 × 3 cm low attention lesion of the left lobe of the liver and a small low attenuation lesion of the spleen; however, this occurred at an outside institution and unfortunately, there was no follow-up on these results. After spontaneous rupture of the membranes she had an uncomplicated vaginal delivery at 37 weeks. The newborn infant male was noted to have pallor; CBC demonstrated a hematocrit of 21. The anemia was thought to be due to a fetomaternal hemorrhage and he received a blood transfusion. His hemoglobin levels returned to normal, and he and his mother were discharged home on postpartum day 2.
The mother returned approximately one month later with debilitating shoulder pain, shortness of breath and abdominal distension. She was immediately transferred via helicopter to our institution, where she would be diagnosed and treated. CT scan revealed innumerable lesions in the lung, liver lesions now measuring 12.6 cm and with evidence of subcapsular hematoma surrounding the left lobe of the liver (Fig. 1), and the spleen containing a 5.4 cm enhancing lesion as well. Hemoperitoneum was noted. β-hCG level was obtained and was found to be 4.9 million mIU/ml. A diagnosis of metastatic choriocarcinoma was made based on the clinical presentation, CT findings and elevated serum β-hCG.
Fig. 1.

CT scan of the mother showing a 12.6 cm liver lesion and subcapsular hematoma surrounding the left lobe of the liver.
Upon the diagnosis of the mother, the infant was brought back to the hospital and was found to have a β-hCG level of 339,000 mIU/ml. CT scan showed a 4.5 × 3.5 cm liver mass (Fig. 2), eight lung lesions (the largest measuring 3 cm), as well as bilateral ocular lesions. Physical exam revealed his liver palpable 3 cm below the costal margin. Based on these findings, the diagnosis in the mother and elevated β-hCG levels, the diagnosis of disseminated infantile choriocarcinoma was made. It was determined that the risks of tissue biopsy including excessive bleeding outweighed the potential benefits.
Fig. 2.
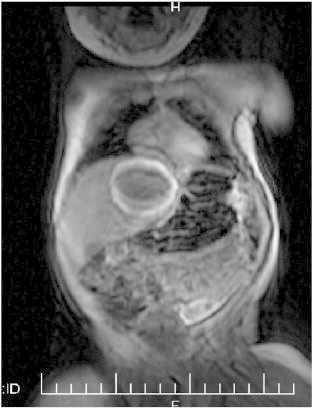
CT scan of the baby showing a 4.5 × 3.5 cm liver mass.
Treatment of choriocarcinoma in the mother
After diagnosis, the patient underwent an emergent selective hepatic embolization and splenic artery embolization, as well as multiple blood transfusions. A MRI of the brain was performed at that time and was highly suspicious for brain metastasis. During her hospital stay she was given a dose of Cisplatin and Etoposide (EP) on hospital day 12. She was discharged on hospital day 19 and her β-hCG at that time was 15,000. The patient was started on Etoposide, Dactinomycin and Methotrexate (EMA) and Cisplatin and Etoposide (EP) chemotherapy regiment with EMA and EP alternated on a weekly basis for 6 weeks.
Approximately one year after delivery, the patient's β-hCG level was < 2 mIU/ml; she was feeling well, and had no significant complaints other than easy fatigability, paresthesias of her fingers and toes, and some hypoacusia, which seemed to be gradually improving. Two years post-diagnosis, she still shows no evidence of disease, and is currently on a birth control pill.
Treatment of choriocarcinoma in the infant
The patient began chemotherapy immediately after diagnosis. The first 5 cycles consisted of Bleomycin (.5 units/kg, 2 units, single dose), Etoposide (3 mg/kg daily × 5 days), and Cisplatin (.7 mg/kg daily × 5 days) based on the Children's Cancer Group (CCG) protocol 8891 for germ cell tumors (Rogers et al., 2004). Audiometry tests after the fifth cycle indicated high frequency hearing loss, so HDMTX was given for the remaining 3 cycles as follows: Methotrexate (33 mg/kg, single dose) and Leucovorin (15 mg/m2). His β-hCG levels were monitored throughout the treatment and decreased to normal (< 2 mIU/ml) after 5 cycles. In total he received 8 cycles of chemotherapy on 21-day intervals.
He tolerated chemotherapy well with few complications. Following the first cycle, there was significant bleeding into the liver mass, causing it to double in size. After the second cycle, he suffered severe adrenal insufficiency due to high dose dexamethsone (used as an anti-emetic), accompanied by severe hypoglycemia (glucose 10 mg/dl) and a serum cortisol level of 0. He received cortisol supplements for over two months until his levels returned to normal.
His most recent MRI showed complete resolution of all lung metastases and marked reduction in the size of the liver mass and eye lesions. Audiometry testing still showed high frequency hearing loss. Two years later, he continues to show no evidence of disease with a negative β-hCG.
Comment
Choriocarcinoma, amongst other forms of gestational trophoblastic neoplasia (GTN), has been described at length in medical literature. Maternal choriocarcinoma is extremely rare, occurring in an estimated 1 in 50,000 live births (Tidy et al., 1995). Infantile choriocarcinoma is even more rare; less than 30 cases have been described in the literature (van der Hoef et al., 2004). Newborn infants tend to present with a characteristic clinical picture of anemia, hepatomegaly, and precocious puberty (Blohm et al., 2001). Regardless of variation in clinical presentation, β-hCG is raised in serum and/or urine due to the trophoblastic origin of tumor cells (van der Hoef et al., 2004).
Here we report the successful treatment of choriocarcinoma in both mother and infant. In both patients, diagnosis was made based on CT scans, elevated serum β-hCG, and clinical findings. Approximately one-quarter of infants with choriocarcinoma present with symptoms at birth and diagnosis can be easily confirmed with a serum β-hCG (McNally et al., 2002). In our case, the diagnosis was easily reached based on the clinical presentation of the mother. However, in the rare instances when the mother has no GTN, infantile choriocarcinoma can be easily misdiagnosed or missed. If an infant presents with anemia and a liver mass, as did our patient, serum β-hCG levels are critical for diagnosis because isolated elevation of β-hCG in the presence of liver mass has been reported only in choriocarcinoma. β-hCG levels can be followed throughout the treatment to assess the response to chemotherapy, and to detect recurrences in both mother and child. These tumors are highly vascular and friable, so biopsy may be difficult and even dangerous (Yoon et al., 2007). As such, in the infant we describe, treatment begun immediately without tumor biopsy.
Choriocarcinoma is a very aggressive malignancy and death may result from delays in diagnosis. Therefore, early intervention – which can be difficult because of the rarity of the disease – is critical for limiting the progression of disease. In infants, without appropriate treatment, death usually occurs within 3 weeks of initial presentation (Yoon et al., 2007). Fortunately, despite its aggressive nature, this cancer responds very well to chemotherapeutic agents, even in the presence of widespread metastases. The chance of long-term survival, even in patients with cerebral metastases at presentation, is approximately 80% (McNally et al., 2002). Both patients described responded very well to chemotherapy, without significant complications, and currently have shown no progression or recurrence of disease in the two years since diagnosis.
Patient neglect is never excusable, especially with the signs and symptoms the mother presented with to her original physicians. However, this instance does provide us with some education; the fact that the tumor had progressed further and still resolved with treatment speaks to this aggressive cancer's excellent response to chemotherapy.
After completing her treatment, the mother was advised to begin using birth control pills. Because a history of maternal choriocarcinoma is associated with a risk of infantile choriocarcinoma in subsequent pregnancies, current guidelines suggest that women with such a history should be checked for β-hCG at 6 and 10 weeks following a subsequent pregnancy, regardless of the outcome (Blohm et al., 2001; McNally et al., 2002).
Conflict of interest statement
No conflict of interest.
References
- Berkowitz R.S., Goldstein D.P. Pathogenesis of gestational trophoblastic neoplasms. Pathobiol. Annu. 1981;11:391. [PubMed] [Google Scholar]
- Blohm M.E. Disseminated choriocarcinoma in infancy is curable by chemotherapy and delayed tumour resection. Eur. J. Cancer. 2001;37(1):72–78. doi: 10.1016/s0959-8049(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Loukovaara M. Epidemiology of hydatidiform mole in Finland, 1975 to 2001. Eur. J. Gynaecol. Oncol. 2005;26(2):207–208. [PubMed] [Google Scholar]
- McNally O.M. Successful treatment of mother and baby with metastatic choriocarcinoma. Int. J. Gynecol. Cancer. 2002;12(4):394–398. doi: 10.1046/j.1525-1438.2002.01125.x. [DOI] [PubMed] [Google Scholar]
- Rogers P.C., Olson T.A., Cullen J.W. Treatment of children and adolescents with stage II testicular and stage I and II ovarian malignant germ cell tumors: a pediatric intergroup study — Pediatric Oncology Group 9048 and Children's Cancer Group 8891. J. Clin. Oncol. 2004;22:3563–3569. doi: 10.1200/JCO.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Tidy J.A. Presentation and management of choriocarcinoma after nonmolar pregnancy. Br. J. Obstet. Gynaecol. 1995;102(9):715–719. doi: 10.1111/j.1471-0528.1995.tb11429.x. [DOI] [PubMed] [Google Scholar]
- van der Hoef M. Solitary infantile choriocarcinoma of the liver: MRI findings. Pediatr. Radiol. 2004;34(10):820–823. doi: 10.1007/s00247-004-1212-x. [DOI] [PubMed] [Google Scholar]
- Yoon J.M. Treatment of infantile choriocarcinoma of the liver. Pediatr. Blood Cancer. 2007;49(1):99–102. doi: 10.1002/pbc.20623. [DOI] [PubMed] [Google Scholar]


