Highlights
► We report a case of recurrent vulvar carcinoma with a good response to erlotinib. ► Treatment was well tolerated with no serious side effects. ► Further evaluation of this new therapeutic approach may be warranted.
Keywords: EGFR, Erlotinib, Vulvar carcinoma
Introduction
Cancer of the vulva is a rare female genital neoplasm which represents 2.5% of cancer cases among women and 5% of all gynecological malignancies (Hacker, 2000). It occurs most frequently in women between the ages of 65 and 75 and the most common histological type is squamous cell carcinoma followed by melanoma, basal cell carcinoma and adenocarcinoma (Hacker, 2000). Standard therapy for local vulvar carcinoma includes radical local excision with or without inguino-femoral lymphadenectomy, combined with radiotherapy or chemoradiation for locally advanced or unresectable disease. The prognosis is generally good when using this multimodality approach; however, treatments are associated with significant morbidity and 40%–50% will eventually develop recurrence (Hacker, 2000; Lupi et al., 1996).
Epidermal growth factor receptor (EGFR) is over-expressed in a variety of cancers, including both primary vulvar squamous cell carcinoma and metastatic lesions (Johnson et al., 1997). EGFR is a cellular transmembrane receptor activated by the binding of EGF or another growth factor. Activating mutations and amplification of the EGFR induce intrinsic tyrosine kinase activity and cellular signaling that results in cell growth, proliferation, invasion, angiogenesis, metastasis and inhibition of cell death (Henson and Gibson, 2006).
Erlotinib (Tarceva®) is an oral, reversible EGFR tyrosine-kinase inhibitor. The drug received US Food and Drug Administration approval for the treatment of non-small cell lung cancer in 2004 and for treatment of patients with pancreatic cancer in 2005. The first experience with erlotinib in the treatment of two elderly patients with locally advanced vulvar cancer was reported in 2007; in both cases, dramatic responses were observed (Olawaiye et al., 2007).
In this report, we describe the case of a patient with a recurrent squamous cell vulvar carcinoma after surgery and chemoradiation who responded to erlotinib during 9 months with excellent tolerability.
Case presentation
A 76-year old woman presented to the hospital with a six-month history of a left vulvar lesion. Hypertension well controlled was her main co-morbidity. Clinical examination showed a 12 cm exophytic left vulvar mass (Fig. 1). CT-scan and MRI showed a heterogeneous vulvar mass with left inguinal lymph node involvement. Diagnosis was established on a biopsy of the mass showing an infiltrating well differentiated squamous cell carcinoma. The tumor was at least FIGO (International Federation of Gynecology and Obstetrics) stage IIIA. The patient underwent two cycles of induction chemotherapy with fluorouracil and cisplatin before chemoradiation; the latter was combined with a daily fraction of radiotherapy (total dose 46 Gy) and concurrent weekly carboplatin. The tumor and lymph nodes shrank by 80% and 40% respectively. Six weeks later, a radical left hemivulvectomy with homolateral lymph node dissection was performed. Post-operative histology showed important signs of a regression of the primary (> 50%) and only one lymph node involved out of five. Eleven months after surgery, the patient presented with a large local recurrence not amenable to surgery. Two cycles of 5-flurouracil and cisplatin were delivered but could not prevent disease progression. Symptoms included perineal pain, bleeding, inability to sit and loss of weight.
Fig. 1.
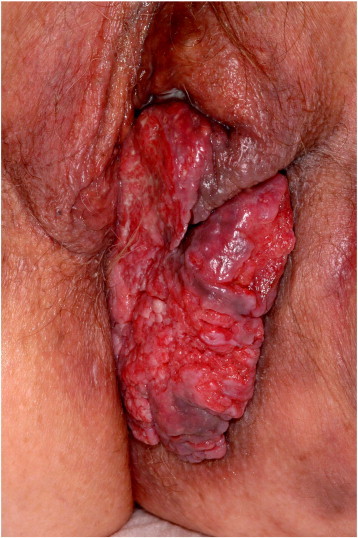
Exophytic left vulvar mass.
The patient started erlotinib at a dosage of 150 mg daily. The tumor remained stable for 9 months with significant improvement of pain and bleeding and decrease of the uptake of analgesics. The patient could sit again. Toxicity included grade 2 skin rash. Evaluation after 9 months of treatment showed eventually a clear disease progression and erlotinib was stopped. The patient died 1 month later under symptomatic cares.
Discussion
In locally advanced vulvar cancer, recurrence occurs in about half of the patients after primary treatment including surgery with or without chemoradiation (Lupi et al., 1996). Both treatment and outcome depend on the site and extent of recurrence. Local recurrences without regional node involvement can be managed successfully in many instances by repeated local excision and/or radiation therapy providing an approximate 5-year survival rate of 56%. Recurrent lesions in the lymph node area not amenable to surgery or radiotherapy, as well as in distant sites, are more difficult to treat, and the 5-year survival rate is generally less than 5% (Lupi et al., 1996).
The role of chemotherapy, in this setting, is very limited and the goal is only palliative. Drugs that have been used include cisplatin, bleomycin, methotrexate and more recently paclitaxel and vinorelbine with minimal activity in frequently heavily pretreated patients (Deppe et al., 1979). There is clearly a need for more effective therapeutic approaches. Using an EGFR inhibitor like erlotinib in vulvar cancer supported by the known EGFR overexpression which occurs in 60–70% of primary vulvar squamous cell carcinoma is associated with a poor outcome. Johnson et al. reported that there is a progressive increase in EGFR expression in a similar patient, from benign vulvar epithelium (31%) to primary malignant tissue (65%) and then to metastatic lesions (88%). The 5-year disease free survival was significantly worse when EGFR levels in the primary tumor was ≥ 90% vs < 90%: (25% vs 54% respectively—p < 0.05) (Oonk et al., 2007). Additionally, in the study by Oonk et al. EGFR expression was present in 68% of vulvar tumors and was related to the presence of lymph node metastases (Oonk et al., 2007). More recently, the amplification of the EGFR gene was reported as conferring a decreased survival, in a cohort of 51 vulvar cancer patients (Growdon et al., 2008).
In 2007, Olawaiye et al. published the first two cases of locally advanced squamous cell vulvar carcinoma treated with erlotinib at a dosage of 150 mg daily. Dramatic responses were observed in both cases before the third week of treatment but both patients died of several co-morbidities and the duration of responses was limited (1 and 2 months) (Olawaiye et al., 2007). In our case, the tumor remained stable for 9 months with significant palliation of symptoms. To our best knowledge, this is the longest response published of a case of vulvar carcinoma treated with erlotinib.
Erlotinib is an oral medication that can be taken at home by older patients with decreased cost and hospital stay and a good tolerability. In this view, it appears as an attractive alternative treatment for advanced vulvar carcinoma. Other EGFR inhibitors have been investigated for treatment of vulvar squamous cell carcinoma. A combination of cetuximab (anti-EGFR monoclonal antibody) and cisplatin was efficient in one patient with recurrent metastatic vulvar carcinoma (Richard et al., 2008). Gefitinib, another EGFR inhibitor combined with trastuzumab, an anti-HER2 (human epidermal growth factor receptor 2) has been investigated in a human vulvar carcinoma cell line (A431), and seems to increase radiosensitivity (Fukutome et al., 2006).
Conclusion
As shown in this case report, the potential effect of erlotinib for vulvar carcinoma, seems to be an important area of research for this disease and deserves further evaluation in well designed studies.
Conflict of interest statement
The authors declare that they have no competing interests.
Footnotes
The authors declare that they have no financial support and no competing interests.
References
- Deppe G., Cohen C.J., Bruckner H.W. Chemotherapy of squamous cell carcinoma of the vulva: a review. Gynecol. Oncol. 1979;7:345–348. doi: 10.1016/0090-8258(79)90112-4. [DOI] [PubMed] [Google Scholar]
- Fukutome M., Maebayashi K., Nasu S. Enhancement of radiosensitivity by dual inhibition of the HER family with ZD1839 (“Iressa”) and trastuzumab (“Herceptin”) Int. J. Radiat. Oncol. Biol. Phys. 2006;66(2):528–536. doi: 10.1016/j.ijrobp.2006.05.036. Oct 1. [DOI] [PubMed] [Google Scholar]
- Growdon W.B., Boisvert S.L., Akhavanfard S. Decreased survival in EGFR gene amplified vulvar carcinoma. Gynecol. Oncol. 2008;111(2):289–297. doi: 10.1016/j.ygyno.2008.07.038. Nov. [DOI] [PubMed] [Google Scholar]
- Hacker N.F. Vulvar cancer. In: Berek J.S., Hacker N.F., editors. Practical Gynecologic Oncology. 3d ed. Williams & Wilkins; Philadelphia: 2000. pp. 553–596. [Google Scholar]
- Henson E.S., Gibson S.B. Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell. Signal. 2006;18(12):208997. doi: 10.1016/j.cellsig.2006.05.015. Dec. [DOI] [PubMed] [Google Scholar]
- Johnson G.A., Mannel R., Khalifa M. Epidermal growth factor receptor in vulvar malignancies and its relationship to metastasis and patient survival. Gynecol. Oncol. 1997;65(3):425–429. doi: 10.1006/gyno.1997.4660. Jun. [DOI] [PubMed] [Google Scholar]
- Lupi G., Raspaliesi F., Zucali R. Combined preoperative chemoradiotherapy followed by radical surgery in locally advanced vulvar carcinoma. Cancer. 1996;77:1472–1478. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1472::AID-CNCR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Olawaiye A., Lee L.M., Krasner C. Treatment of squamous cell vulvar cancer with the anti-EGFR tyrosine kinase inhibitor Tarceva. Gynecol. Oncol. 2007;106(3):628–630. doi: 10.1016/j.ygyno.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Oonk M.H., de Bock G.H., van der Veen D.J. EGFR expression is associated with groin node metastases in vulvar cancer, but does not improve their prediction. Gynecol. Oncol. 2007;104(1):109–113. doi: 10.1016/j.ygyno.2006.07.035. Jan. [DOI] [PubMed] [Google Scholar]
- Richard S.D., Krivak T.C., Beriwal S. Recurrent metastatic vulvar carcinoma treated with cisplatin plus cetuximab. Int. J. Gynecol. Cancer. 2008;18(5):1132–1135. doi: 10.1111/j.1525-1438.2007.01145.x. Sep–Oct. [DOI] [PubMed] [Google Scholar]


