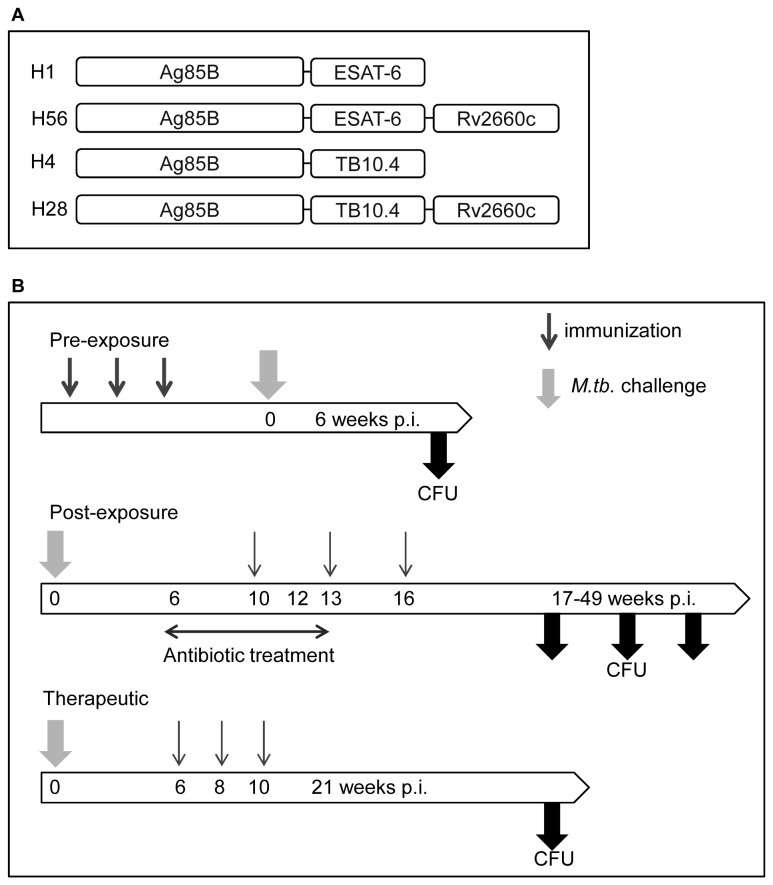
Figure 2. The vaccine constructs and animal challenge models.
(A) A schematic overview of the multi-component vaccine constructs which include the ESAT-6-containing vaccines: H1 (Ag85B-ESAT-6) and H56 (Ag85B-ESAT-6-Rv2660c), and the TB10.4-containing vaccines: H4 (Ag85B-TB10.4) and H28 (Ag85B-TB10.4-Rv2660c). (B) Experimental setups for the pre-exposure, post-exposure and therapeutic models. Pre-exposure model: Mice receive three immunizations with a two week interval between each immunization. Six weeks after the last immunization, mice were aerosol challenged with 100 CFU of M.tb. Erdman. Six weeks post-infection (p.i.) mice were sacrificed and CFU levels monitored in the lungs. Post-exposure model: Mice receive an aerosol challenge with 50 CFU of M.tb. Erdman. After six weeks of infection, antibiotics are administered through the drinking water for a six week period. Vaccinations are given at week 10, 13, and 16 after infection. CFU levels in the lungs are monitored between week 17 and 49 p.i. Therapeutic model: Mice receive an aerosol challenge with 50 CFU of M.tb. Erdman. The mice receive three immunizations with two weeks interval (starting at week 6 p.i.), and the CFU levels in the lungs are monitored at week 21 p.i.