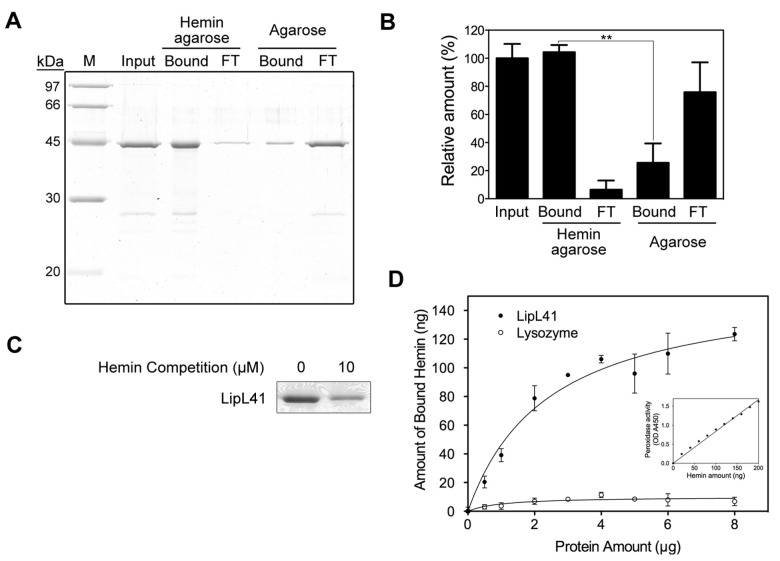
Figure 4. Hemin-binding ability of LipL41.
(A) The hemin binding of LipL41 was examined by hemin-agarose beads. Shown are input sample (Input), hemin-agarose-bound (Bound) and resulting flow through (FT), and the non-specific binding control of agarose-bound (Bound) and resulting flow through (FT). Agarose without hemin was the non-specific binding control. The experiments were repeated three times independently. (B) The results from (A) were quantitated by ImageJ software and the difference of binding to hemin-agarose and to agarose alone was determined by Student’s t-test (**: p-value < 0.01). (C) LipL41 was incubated with 0 or 10 μM hemin prior to binding with hemin-agarose beads. The hemin-binding ability of LipL41 was competed by preincubating with hemin. (D) The amount of hemin bound by LipL41 was estimated by inherent hemin-dependent peroxidase activity. A microtiter plate was coated with various protein amounts (0-8 μg) of LipL41 (solid symbol). Lysozyme was the negative control (open symbol). The peroxidase activity of the bound hemin was assayed by the addition of tetramethylbenzidine and reading of the absorbance at 450 nm. The inset shows the standard graph of peroxidase activity of hemin. The amount of bound hemin was increased until about 3 μg of LipL41, when the hemin reached a plateau level of about 100 ng of hemin with a molar ratio of 0.5 (LipL41 to hemin). The K d was calculated to be 0.59 ± 0.14 μM. The statistical significance of hemin binding was determined by Student’s t-test with a p-value of 0.001.