Abstract
Arthrospira (Spirulina) platensis (ASP) is a representative filamentous, non-N2-fixing cyanobacterium that has great potential to enhance the food supply and possesses several valuable physiological features. ASP tolerates high and low temperatures along with highly alkaline and salty environments, and can strongly resist oxidation and irradiation. Based on genomic sequencing of ASP, we compared the protein expression profiles of this organism under different temperature conditions (15°C, 35°Cand 45°C) using 2-DE and peptide mass fingerprinting techniques. A total of 122 proteins having a significant differential expression response to temperature were retrieved. Of the positively expressed proteins, the homologies of 116 ASP proteins were found in Arthrospira (81 proteins in Arthrospira platensis str. Paraca and 35 in Arthrospira maxima CS-328). The other 6 proteins have high homology with other microorganisms. We classified the 122 differentially expressed positive proteins into 14 functions using the COG database, and characterized their respective KEGG metabolism pathways. The results demonstrated that these differentially expressed proteins are mainly involved in post-translational modification (protein turnover, chaperones), energy metabolism (photosynthesis, respiratory electron transport), translation (ribosomal structure and biogenesis) and carbohydrate transport and metabolism. Others proteins were related to amino acid transport and metabolism, cell envelope biogenesis, coenzyme metabolism and signal transduction mechanisms. Results implied that these proteins can perform predictable roles in rendering ASP resistance against low and high temperatures. Subsequently, we determined the transcription level of 38 genes in vivo in response to temperature and identified them by qRT-PCR. We found that the 26 differentially expressed proteins, representing 68.4% of the total target genes, maintained consistency between transcription and translation levels. The remaining 12 genes showed inconsistent protein expression with transcription level and accounted for 31.6% of the total target genes.
Introduction
Arthrospira (Spirulina) platensis (ASP) is a genera of filamentous, non-heterocyst-forming cyanobacteria. It is gram-negative, has no flagellum and contains chlorophyll-a that carries out photosynthesis producing oxygen. It is well known for its useful application as a food source [1]. ASP possesses abundant proteins representing 60-70% of its dry weight and its protein composition is nearly perfect for human nutrition. It contains eight necessary amino acids, various unsaturated fatty acids, vitamins, mineral substances and biologically active agents which are necessary for the human body. These substances provide increased immunity, anti-cancer and anti-aging benefits, radiation resistance, antioxidant properties, and reduction to drug toxicity effects. Consequently, ASP is widely applied to several fields, such as functional food, compound feedstuff, new model medicines and fine chemicals [2].
Scientists have found that this organism also possesses some unusual but valuable physiological features, such as adaptation to highly alkaline environments, resistance to high or low temperatures, antioxidant activity, and radiation and salt tolerance. Due to these properties, it often occupies a dominant ecological position in lakes with high carbonate/bicarbonate levels. One of the most common stresses for many living organisms is extreme variation in their surrounding temperature. Adaptive mechanisms that protect against the potentially harmful effects of elevated or reduced temperatures are found in all living organisms. These survival pathways are primarily regulated at the transcription level and then at post-transcription and translation levels [3].
In recent years, heat-shock or cold-shock response in some cyanobacteria, such as Synechocystis, Synechococcus and Nostoc, has been investigated [4–6]. Kurdrid et al. [7] demonstrated that the heat-shock protein (Hsp) and an alternative sigma factor (SigH) in Spirulina plantensis were significantly induced immediately after exposure to high temperature. Moreover, expression of the HspA gene in Synechococcus was controlled via the binding of regulatory proteins to the upstream AT-rich region [8]. Immunocytochemical studies by Kojima showed that the main localization of HspA in the cyanobacterium Synechococcus strain ECT16-1 cells shifted from the thylakoid area to the cytoplasm, then back to the thylakoid area during heat stress [8]. Expression of HspA stabilizes the morphology of nucleoids and this unique property of HspA is associated with thylakoid membranes [7]. Balogi et al. [9] observed an increase in the level of the highly saturated monoglucosyldiacylglycerol (MGlcDG) in Synechocystis cells. The MGlcDG membranes remain stable even at extremely high temperatures (45°C) and express the strongest interaction with the thylakoid-stabilizing Hsp17 from Synechocystis. They suggest that the highly saturated MGlcDG functions as a “heat-shock lipid” and is of potential importance in the development of acquired thermotolerance of heat/light-primed cyanobacterial thylakoids [9]. Similarly, the desD gene that encodes Δ6-desaturase of Spirulina platensis is regulated at the transcription level in response to temperature reduction. This regulation occurs via a protein complex, consisting of the GntR regulatory protein and the GroEL chaperone [10]. The effects of low and high temperatures on the transcription of these desaturase genes in Spirulina have been previously reported. A temperature change from 35 to 40°C in the presence of light does not significantly affect either the mRNA levels of desC, desA and desD genes or the stability of the desD mRNA [11].
At present, due to the lack of whole-genome sequences for ASP, proteomics research examining environmental stress is limited. As a result, proteome studies of ASP are still based on sub-cell levels, such as the thylakoid membrane, plasma membrane, etc. [12]. In addition, much of the previous research has focused on the search for specific genes or regulating factors, while little data are available on gene functional research using a systematic approach [13,14,15]. This paper aimed to analyze the protein expression of ASP using 2-dimensional gel electrophoresis (2-DE) and MALDI-TOF/MS to further explore temperature response, track the differential expression spectrum, identify the differentially expressed proteins, and analyze their main physiological functional classification. On this basis, we discussed the resistance mechanisms of ASP under different temperature conditions. Additionally, we used qRT-PCR to validate the consistency of subset genes between transcription and translation levels. This research can inform a better understanding of environmental adaptation mechanisms of cyanobacteria, and provides basic clues to further develop ASP’s gene resources, which have the ability to tolerate several adverse environmental conditions.
Material and Methods
Culture conditions and sample preparation
ASP-YZ supplied by the Chinese Academy of Agricultural Sciences (Beijing, China) was cultured in 0.02 M NaCl Zarrouk medium at 35°C, 8 klx intensity and 75% humidity [16]. At the logarithmic growth period, ASP-YZ cells were transferred into fresh Zarrouk medium (initial inoculated concentration of OD560=0.2) at three different temperatures (15°C for low temperature, 35°C for control and 45°C for high temperature). Three biological replicates were established for each temperature treatment, i.e., three conical flasks of ASP (500 mL/flask) were cultured at each temperature (15, 35 or 45°C) for 7 d. Then, we collected the filaments from each flask. The harvested cells were washed three times with phosphate-based buffer, and then centrifuged at 8,000 rpm for 5 min to pellet old and dead cells. The cells in the supernatant were washed five times with ultra-pure water to ensure complete removal of salt. Finally, 9 individual filamentous samples were obtained, and stored at -80°C for further protein extraction.
Protein extraction
First, the 9 individual filamentous samples prepared as described above were immediately frozen in liquid nitrogen for 10-15 min. Then, they were sedimented using trichloroacetic acid (TCA)/acetone as described in Giaralisco et al. [17], which resulted in protease inactivation, inhibition of protein degradation, and removal of impurities and extremely alkaline proteins. This TCA/acetone sediment technique is a routine method for extracting protein from cyanobacteria. After centrifugation, the precipitate was ultrasonically treated for 12 min in ice and resuspended with acetone, containing 0.07% BME at -20°C for 1-2 h, to fully remove pigments, then centrifuged at 35,000 g and 4°C for 15 min, and finally vacuum-dried. The protein pellets were then dissolved in an improved dissolving buffer, which contained 2 M thiourea, 5 M urea, 40 mM Tris, 20 mM DTT, 5 mM TCEP, 1% IPG buffer pH 3-10 (GE-Healthcare Biosciences, USA), 0.05% β-dodecyl maltoside and 2% CHAPS. The cell debris was discarded following centrifugation at 35,000 g for 30 min at 4°C. For expediency with subsequent experiments, the extracted proteins from the three biological replicates of each temperature treatment were composited into one sample. As a result, the 9 individual filamentous samples originating from the three temperature treatments yielded three mixed protein samples. The mixed protein concentration of the composited samples was determined using the DC-kit protein assay (Bio-Rad, USA) [7]. Finally, these protein samples were aliquoted, immediately frozen in liquid nitrogen and stored at -30 °C for later use.
Two-dimensional gel electrophoresis
For one-DE, three technical replicates were established for each composited protein sample. The protein extract (22 µL) was diluted to a final concentration of 1000 µg/ml with an IEF rehydration solution, consisting of 17.5 µL DTT, 1.75 µL IPG (immobilized pH gradient buffer solution) and 309 µL R-Buffer (2 M sulfourea, 7 M urea, 4% CHAPS, 40 mM Tris, 2 mM TBP reductant and 0.2% Biolyte). Then, the above diluted protein extract (350 µL) was subjected to Immobiline DryStrips (pH 4-7; Length 17 cm; Amersham Bioscience). In a preliminary experiment, the protein isoelectric points (pI) for most Spirulina species were found to be pH 4-7 by means of drystrips in the pH range of 3-10. Therefore, the maximum separation was obtained by using the pH 4-7 drystrips. For the first-dimensional IEF (isoelectric focusing) based on the pI value of each protein, the procedures were performed as described by Blum et al. [18]. After completing the above IEF, 9 drystrips were used for electrophoresis in parallel using 12% polyacrylamid to produce 9 gels. The proteins on the gels were stained with the silver-based color development system using Gelcode (The Upjohn Co.). In order to improve the dynamic range of protein quantification in silver-stained gel and make it possible to visualize both high- and low-abundance proteins on a 2-DE gel, pre-processing of images was performed as described in Grove et al. [19]. Gels were fixed overnight in 50% ethanol/5% acetic acid, and rinsed (5 gels/tray) four times (1 h per rinse) in 1,800 ml of ultra-pure water. Following washing, the gels were equilibrated in 1.9 g/L AgNO3 (1 L/tray) for 60-90 min. An additional 1-h wash in Na2CO3 (7.5 g/L) followed the reducing step [7]. The protein spots were scanned using a UNAX Powerlook 2100XL (Bio-Rad) and analyzed by ImageMaster 2D platinum 5.0 (Amersham Bioscience) according to manufacturer’s instructions.
Image acquisition and data analysis
Images were cropped and optimized before performing the inter-gel matching of the standard protein maps. Before spot matching, the internal standard image gel with the greatest number of spots was used as a master gel. The spot detection parameters were optimized by checking different protein spots in certain regions of the gel, which were then automatically detected, followed by visual inspection for removal or addition of undetected spots. The TwoClassDif (RVM-T test) method with a cutoff p-value <0.05 and FDR <0.05 was applied to analyze the differentially expressed proteins in the three different temperature treatments. Spot detection was refined by a manual spot edition where required. The spots that were visible on at least two gels from each temperature treatment or control based on the image analysis were identified as stably expressed protein spots. The abundance of each protein spot was estimated by the percentage volume (Vol%), that is, the spot volumes were normalized as a percentage of the total protein volume in all spots visible in the gel to correct for variability due to loading, gel staining and de-staining [20,21]. The percentage volumes were used to designate significant differential expression spots (at least three-fold increase/decrease and statistical significance as calculated by the RVM-T test with a cutoff p-value <0.05 and FDR <0.05). Triplicate gels were used for each sample. Only those with reproducible and significant changes were considered to be differentially expressed protein spots. Data were reported as mean ± standard deviation (mean ± SD), which was calculated by SPSS 16.0 software (SPSS, Chicago, IL, USA). The solely visible or not visible protein spots, scanned by UNAX Powerlook 2100XL, were judged by image analysis in a compared experimental group, such as 15°C vs 35°C, 45°C vs 35°C or 15°C vs 45°C. The significant differentially expressed protein spots were judged using the criterion >3-fold up-regulation or down-regulation.
Protein identification by peptide mass fingerprinting (PMF) and MALDI-TOF MS analysis
The differentially expressed protein spots were manually selected and excised, then de-stained in 50% ACN in 25 mmol/L NH4HCO3 at 37°C for 30 min. Next, 50 µL ultra-pure H2O and 50 µL 50% ACN were added, followed by 100 µL of 100% ACN. The gels were rehydrated in 5 µL of trypsin (Promega, Madison, USA) solution (20 µg/ml in 25 mmol/L NH4HCO3) for 30 min. Next, 20 µL of cover solution (25 mmol/L NH4HCO3) was added, and digestion took place over night at 37°C. The supernatant was transferred into another tube, and the gels were extracted once with 50 µL extraction buffer (67% ACN and 5% TFA). The peptide extracts and the supernatant of the gel spot were combined and then completely dried. Samples were re-suspended in 5 µL 0.1% TFA followed by mixing in a 1:1 ratio with a matrix consisting of a saturated solution of CHCA in 50% ACN containing 0.1% TFA. The 1:1 mixture was spotted on a stainless steel sample target plate. The peptide samples were analyzed with a MALDI-TOF-MS Proteomics Analyzer (Bruker Daltonics, Bremen, Germany). The TOF spectra were recorded in positive ion reflector mode with a mass range from 800 to 4000 Da. About eight subspectra with 60 scans per subspectrum were accumulated to generate one main TOF spectrum. Data were searched on the Internet using a Mascot search engine (Matrix Science Ltd., London, UK) against all entries in the NCBInr database, which contained accessible public protein sequences (http://www.ncbi.nlm.nih.gov/nuccore/NZ_ACSK01001820.1/ Arthrospira platensis str. Paraca NZ_ACSK01001820 and http://www.ncbi.nlm.nih.gov/nuccore/NZ_ABYK00000000.1/ Arthrospira maxima CS-328), and the unpublished Arthrospira (Spirulina) database. The unpublished database was compiled by our group after nearly complete sequencing and annotation of Arthrospira (Spirulina) platensis-YZ originating from Beijing, China (The genome database of ASP-YZ is in the process of submission, but has not yet been published). All peptide masses were assumed monoisotopic and [M+H]+. The other parameters used for search were as follows: taxonomy, other bacteria; enzyme, trypsin; the fixed modification; carbamidomethyl (C); the variable modification, Glu- > pyro-Glu (N-term Q) and oxidation (M); mass toll = ±100 pm. The confidence in the peptide mass fingerprinting matches (p <0.05) was based on the MOWSE Score and confirmed by the accurate overlapping of the matched peptides with the major peaks of the mass spectrum. Only the significant hits, as defined by a MASCOT probability analysis (p <0.05), were accepted. If the theoretical values of MW and pI, which were identified by peptide mass fingerprinting (PMF) and MALDI-TOF/MS analysis, were consistent with the actual values analyzed by ImageMaster 2D platinum 5.0 in gel, we regarded this protein spot as a positive match. The matched peptides of this protein were blasted to the protein database nr (ftp://ftp.ncbi.nih.gov/blast/db/) and Swiss-Prot (ftp://ftp.uniprot.org/pub/databases/uniprot_datafiles_by_format/fasta/) in order to obtain the protein functional annotation information with the highest sequence similarity. Then, we acquired information on the ortholog of gene products, classification of gene functions and metabolic pathway analysis of gene products in cells by KEGG (ftp://ftp.genome.jp/pub/kegg/release/archive/kegg/) and COG (ftp://ftp.ncbi.nih.gov/pub/COG/) (evalue<0.00001) functional classification. These differentially expressed proteins were grouped by their respective metabolic pathways using the COG software online. In this investigation, our research group predicted temperature-responsive genes by metabolic pathway positioning through KEGG analysis, and selected part of the genes for further validation. Some of the selected genes comprised a large proportion of the total differentially expressed genes, and were possible temperature-responsive genes reported in previous reports [22,23,24], while the others were the up-regulated proteins or solely visible spots at 45°C or 15°C, while not visible at 35°C.
qRT-PCR analyses of the differentially expressed genes
Primer sets, shown in Table S1, were designed for amplification of 16S rRNA and differentially expressed genes using Premier 5.0 software, and they were synthesized by Shanghai Sonny Biological Technology Co., Ltd. (Shanghai, China). To validate the differentially expressed proteins of ASP at the transcription level in vivo, qRT-PCR was performed using the StepOneTM RT-PCR System (Applied Biosystem, USA). Three conical flasks of ASP were cultured for each temperature treatment to avoid loss due to pollution or error due to uneven temperatures. We collected the filaments and total RNA was extracted from the ASP-YZ cultured at control (35°C), low (15°C) and high (45°C) temperatures using a RNeasy Plant Mini Kit (Qiagen, Germany) according to manufacturer’s instructions and treated with Dnase I by RNase-Free Dnase Set (Qiagen, Germany). The extracted RNA for each temperature treatment was mixed into one sample, and thus a total of three RNA samples were obtained for the three temperature treatments. The quality of RNA was measured by the Nanodrop method and met the requirements: OD260/OD280 > 1.8 and OD260/OD 230 > 1.5. After the RNA was detected without protein and salt contamination, cDNA was obtained by reverse transcription using a PrimeScript RT-PCR Kit (TaKaRa, Japan), following the procedure composed of an initial denaturation time of 5 min at 95°C, 35 cycles of amplification comprised of a denaturation step for 1 min at 95°C, and the annealing and extension temperatures as listed in Table S2. Each experimental group contained three technical replicates. At the same time, three PCR parallel tubes for control, containing Taq polymerase and extracted RNA as a template, were used to test for DNA contamination. Relative quantification of the targets in each sample was carried out using the signal of 16S rRNA as a stable reference gene [25].
The copy numbers in two samples were first normalized, and the differentially expressed level was then calculated on the basis of the following equation:
Where Gt, Gh, Ct and Ch indicate the target gene of treatment, housekeeping gene of treatment, target gene of control and housekeeping gene of control, respectively.
Results
2-DE profiles of total ASP-YZ proteins in response to different temperatures
The differentially expressed protein profiles of ASP-YZ under three temperature conditions, control (35°C), low (15°C) and high (45°C), were analyzed by 2-DE with triplicate gels for each temperature group (see Figure 1A, 1B, 1C). The overall protein numbers of ASP-YZ were similar when cultured at different temperatures. There were 527 protein spots matched between the control (35°C) and 15°C gels (correlation coefficient (r) of 0.946 and matching rate of 83.7%), 468 between the control and 45°C gels (r = 0.954 and matching rate of 74.7%) and 489 between 15°C and 45°C gels (r = 0.964 and matching rate of 78.7%).
Figure 1. The differential protein spots between low temperature (15°C) treatment and the control (35°C).
Note: The differential protein spots with >3.0-fold changes in low temperature (15°C) treatment compared with the control (35°C).
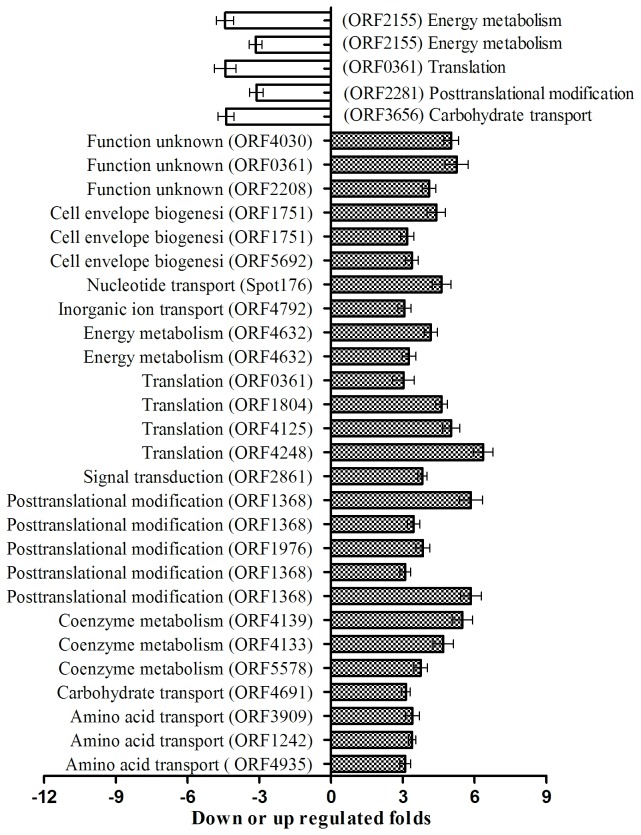
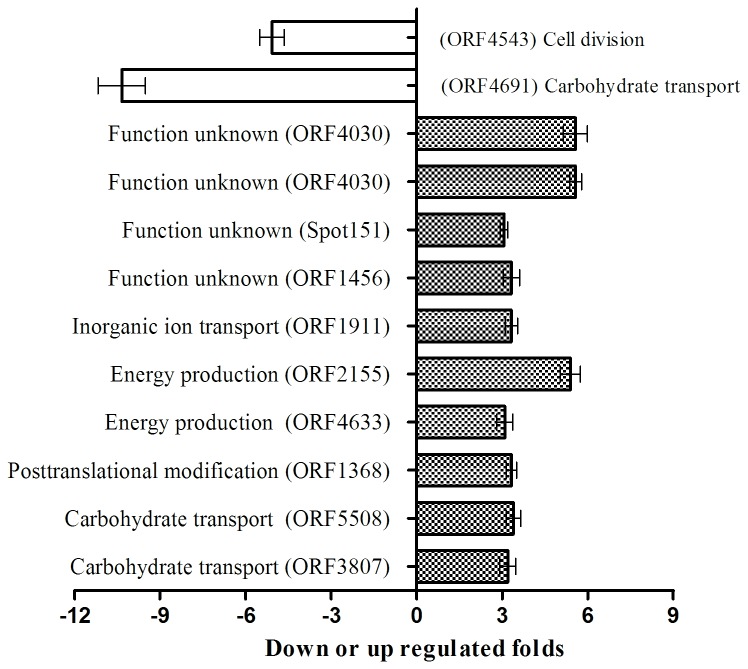
Thirty-two protein spots and their fold changes were found to have more than a 3-fold higher differential expression compared to the control at 15°C (Figure 1). In addition, 18 proteins were solely visible at 15°C, while 21 proteins were solely visible for the control (Table 1). There were 12 protein spots which had over a 3-fold higher differential expression at 45°C compared to the control (Figure 2). In addition, 12 proteins were solely visible for the 45°C treatment, while 8 proteins were solely visible for the control (Table 2).
Table 1. The visible or not visible protein spots at 15°C vs 35°C (control) treatment gels scanned by UNAX Powerlook 2100XL.
| Spot# | Accession NO. | ORF | Gene product | COG Functional classification | MW (kDa,Theor./ Exper.) | pI (Theor./ Exper.) | Matched Peptides | Cov | Score | E-value | Visible or not visible protein spots Vol‰ (Mean± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 395 | ZP_06380542 | ORF0089 | Diaminopimelate epimerase[Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 31.57/32.88 | 4.97/5.04 | 9 | 52% | 94 | 4.00E-04 | -0.66±0.020 |
| 846 | ZP_03272404 | ORF5508 | Ribulose-bisphosphate carboxylase [Arthrospira maxima] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 53.91/53.08 | 6.04/5.19 | 32 | 50% | 228 | 1.70E-17 | +0.78±0.045 |
| 546 | ZP_06381579 | ORF3807 | Glyceraldehyde-3-phosphate dehydrogenase, type I [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 36.89/39.08 | 5.41/5.90 | 15 | 44% | 148 | 1.70E-09 | +1.50±0.091 |
| 847 | ZP_06381851 | ORF4921 | Phosphoglycerate mutase [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 50.68/53.08 | 6.01/6.68 | 14 | 31% | 107 | 2.10E-05 | +0.40±0.062 |
| 46 | ZP_06382410 | ORF4142 | Phosphoglycerate kinase [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 42.36/14.81 | 4.92/5.03 | 6 | 25% | 65 | 3.30E-01 | - 0.64±0.052 |
| 450 | ZP_06382037 | ORF4701 | Pfkb [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 36.86/34.83 | 4.61/4.41 | 8 | 38% | 82 | 7.30E-03 | - 0.60±0.032 |
| 747 | ZP_03272404 | ORF5508 | Ribulose-bisphosphate carboxylase [Arthrospira maxima] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 53.91/46.33 | 6.04/5.78 | 25 | 42% | 230 | 1.00E-17 | - 0.64±0.040 |
| 907 | ZP_06382051 | ORF4691 | Transketolase [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 72.98/62.67 | 5.78/6.16 | 14 | 36% | 148 | 1.60E-09 | - 0.58±0.041 |
| 928 | ZP_06382051 | ORF4691 | Transketolase [Arthrospira platensis] | Amino acid transport and metabolism Carbohydrate transport and metabolism | 72.98/64.76 | 5.78/5.75 | 21 | 45% | 204 | 4.10E-15 | - 1.10±0.087 |
| 306 | YP_002248469 | ORF3841 | Metalloprotease ftsh [Thermodesulfovibrio yellowstonii] | Post-translational modification, protein turnover, chaperones | 66.90/30.37 | 6.12/5.77 | 15 | 26% | 89 | 1.30E-03 | + 0.58±0.046 |
| 60 | ZP_03273444 | ORF3231 | Alkyl hydroperoxide reductase/ Thiol specific antioxidant/ Mal allergen [Arthrospira maxima] | Post-translational modification, protein turnover, chaperones | 15.69/15.45 | 4.66/4.63 | 5 | 42% | 84 | 3.70E-03 | - 1.74±0.032 |
| 805 | ZP_06380920 | ORF1434 | ChaperoninGroEL[Arthrospira platensis] | Post-translational modification, protein turnover, chaperones | 57.43/50.62 | 5.00/4.47 | 24 | 50% | 271 | 8.20E-22 | -1.23±0.021 |
| 806 | ZP_06380920 | ORF1434 | Chaperonin GroEL[Arthrospira platensis] | Post-translational modification, protein turnover, chaperones | 57.43/50.62 | 5.00/4.67 | 26 | 53% | 247 | 2.10E-19 | - 1.18±0.035 |
| 475 | ZP_06381964 | ORF0151 | Hypothetical protein aplap_09815 [Arthrospira platensis] | Signal transduction mechanisms | 9.72 /6.71 | 7.93/6.23 | 9 | 82% | 96 | 2.50E-04 | +0.33±0.023 |
| 451 | ZP_06383067 | ORF0151 | Hypothetical protein aplap_15418 [Arthrospira platensis] | Signal transduction mechanisms | 21.60/35.82 | 5.30/6.57 | 14 | 57% | 99 | 1.40E-04 | + 0.38±0.038 |
| 555 | YP_002485086 | ORF1869 | Multi-sensor signal transduction histidine kinase [Cyanothece sp. PCC 7425] | Signal transduction mechanisms | 85.54/39.52 | 5.95/4.29 | 11 | 12% | 74 | 4.40E-02 | - 0.54±0.042 |
| 480 | ZP_06381260 | ORF4554 | Phage shock protein A, pspa [Arthrospira platensis] | Transcription / Signal transduction mechanisms | 28.18/37.07 | 5.02/4.94 | 10 | 49% | 90 | 9.30E-04 | + 0.68±0.011 |
| 283 | ZP_03271593 | ORF4662 | Sigma 54 modulation protein/ribosomal protein S30EA [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 23.97/29.22 | 7.03/6.22 | 9 | 58% | 94 | 4.00E-04 | - 0.96±0.035 |
| 78 | ZP_06384870 | ORF4030 | Hypothetical protein aplap_24737 [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 17.32/16.31 | 5.96/6.15 | 19 | 86% | 254 | 4.10E-20 | - 0.39±0.047 |
| 89 | ZP_06382587 | / | Hypothetical protein aplap_12998 [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 19.30/16.69 | 9.47/4.11 | 14 | 72% | 187 | 2.10E-13 | - 0.48±0.056 |
| 314 | ZP_06384062 | ORF0361 | Peptidase S8 and S53 subtilisin kexin sedolisin [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 44.24/30.34 | 4.59/4.53 | 16 | 45% | 153 | 5.20E-10 | - 0.64±0.042 |
| 423 | ABV01983 | ORF4633 | Cpch [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 30.85/33.93 | 7.82/6.29 | 25 | 60% | 295 | 3.30E-24 | - 0.42±0.033 |
| 424 | ZP_03272395 | ORF5516 | Conserved hypothetical protein [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 41.12/34.02 | 6.04/6.08 | 13 | 50% | 205 | 3.30E-15 | - 0.58±0.034 |
| 390 | ZP_06382427 | ORF2155 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy metabolism (photosynthesis, respiratory electron transport) | 29.45/33.10 | 9.25/6.61 | 31 | 76% | 313 | 5.20E-26 | + 0.69±0.056 |
| 137 | ZP_03271326 | ORF5159 | Phycobilisome protein [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport) | 17.44/19.04 | 4.89/4.93 | 16 | 62% | 148 | 1.70E-09 | + 0.50±0.066 |
| 140 | ZP_03271568 | ORF4634 | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport) | 17.70/19.59 | 5.82/6.11 | 11 | 75% | 91 | 8.10E-04 | + 0.64±0.043 |
| 147 | ZP_03271568 | ORF4634 | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport) | 17.70/20.47 | 5.82/5.83 | 12 | 75% | 81 | 7.60E-03 | + 0.57±0.054 |
| 47 | ZP_03271327 | ORF4103 | Allophycocyanin, beta subunit [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport) | 17.43/14.81 | 6.26/5.74 | 14 | 84% | 165 | 3.30E-11 | - 0.43±0.007 |
| 318 | ZP_06382427 | ORF2155 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy metabolism (photosynthesis, respiratory electron transport) | 29.45/30.46 | 9.25/4.26 | 15 | 53% | 160 | 1.00E-10 | - 0.52±0.062 |
| 410 | ZP_06382427 | ORF2155 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy metabolism (photosynthesis, respiratory electron transport) | 29.45/33.46 | 9.25/6.41 | 15 | 54% | 186 | 2.60E-13 | - 0.38±0.032 |
| 168 | ZP_06382815 | ORF4792 | DNA starvation/stationary phase protection protein Dps [Arthrospira platensis] | Inorganic ion transport and metabolism | 19.67/23.51 | 4.93/6.24 | 10 | 70% | 81 | 8.50E-03 | + 0.74±0.052 |
| 423 | YP_001999788 | ORF2422 | Preprotein translocase subunit seca [Mycoplasma arthritidis] | Intracellular trafficking and secretion | 99.38/34.57 | 5.10/5.15 | 21 | 28% | 76 | 2.60E-02 | + 0.59±0.034 |
| 540 | ZP_06381727 | ORF2131 | Beta-lactamase-like protein [Arthrospira platensis] | General function prediction only | 34.13/39.06 | 5.12/5.47 | 14 | 40% | 148 | 1.70E-09 | + 0.80±0.033 |
| 10 | ZP_06383388 | ORF2597 | Short-chain dehydrogenase/reductase SDR [Arthrospira platensis] | General function prediction only | 19.51/27.67 | 5.54/6.38 | 12 | 64% | 129 | 1.30E-07 | - 0.40±0.028 |
| 251 | ZP_06381424 | ORF1751 | NAD-dependent epimerase/dehydratase [Arthrospira platensis] | Cell envelope biogenesis, outer membrane | 23.63/28.08 | 5.15/5.75 | 12 | 70% | 184 | 4.10E-13 | - 1.70±0.037 |
| 188 | ZP_06380822 | ORF1456 | Pentapeptide repeat-containing protein [Arthrospira platensis] | Function unknown | 19.86/25.09 | 5.13/5.67 | 16 | 58% | 117 | 2.10E-06 | + 0.67±0.091 |
| 181 | YP_912068 | / | Hypothetical protein Cpha266_1623 [Chlorobium phaeobacteroides] | Function unknown | 31.01/24.60 | 6.56/4.97 | 12 | 43% | 84 | 3.90E-03 | + 0.55±0.12 |
| 391 | ZP_03271502 | ORF4558 | Conserved hypothetical protein [Arthrospira maxima] | Function unknown | 27.65/33.16 | 4.75/4.53 | 11 | 49% | 88 | 1.80E-03 | + 0.32±0.029 |
| 275 | ZP_06380972 | ORF1961 | Ppic-type peptidyl-prolyl cis-trans isomerase | Function unknown | 31.57/28.94 | 5.27/4.94 | 22 | 74% | 190 | 1.00E-13 | + 0.52±0.032 |
Note: (1) In “Visible or not visible protein spots” column, “+”indicates the visible protein spots in the 15°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 15°C treatment gels.
(2) The relative abundance (Vol‰,Mean± SD) of “visible” protein calculated by the ratio of volume of each protein spot to volume of total protein spots in the whole gel.
Figure 2. The differential protein spots between high temperature (45°C) treatment and control (35°C).
Note: The differential protein spots with >3.0-fold changes in high temperature (45°C) treatment group compared to control (35°C).
Table 2. The visible or not visible protein spots at 45°C vs 35°C (control) treatment gels scanned by UNAX Powerlook 2100XL.
| Spot# | Accession NO. | ORF | Gene product | COG Functional classification | MW (kDa,Theor./ Exper.) | pI (Theor./Exper.) | Matched Peptides | Cov | Score | E-value | Visible or not visible protein spots Vol‰ (Mean± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 648 | ZP_03273305 | ORF0278 | Pyridoxal-5'-phosphate-dependent protein beta subunit [Arthrospira maxima] | Amino acid transport and metabolism | 34.84/37.95 | 5.28/5.23 | 26 | 69% | 241 | 8.30E-19 | +0.99±0.031 |
| 331 | ZP_06381552 | ORF0785 | Ribose-5-phosphate isomerase A [Arthrospira platensis] | Carbohydrate transport and metabolism | 25.13/28.28 | 4.96/5.04 | 10 | 46% | 105 | 3.30E-05 | + 0.50±0.045 |
| 1120 | ZP_06381999 | ORF0376 | Transketolase domain protein [Arthrospira platensis] | Carbohydrate transport and metabolism | 69.60/64.11 | 5.19/5.52 | 41 | 64% | 297 | 2.10E-24 | + 0.36±0.085 |
| 129 | ZP_03273475 | ORF1368 | Redoxin domain protein [Arthrospira maxima] | Post-translational modification, protein turnover, chaperones | 19.78/20.11 | 4.88/5.03 | 10 | 49% | 119 | 1.30E-06 | -0.75±0.037 |
| 224 | ZP_03275641 | ORF0073 | Ribosomal protein L6 [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 19.69/25.13 | 10.25/5.63 | 11 | 40% | 104 | 4.20E-05 | +0.60±0.025 |
| 433 | ZP_06380559 | ORF1074 | Elongation factor TS [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 24.63/31.08 | 5.74/5.90 | 21 | 77% | 155 | 3.30E-10 | +0.39±0.019 |
| 283 | ZP_03271593 | ORF4662 | Sigma54 modulation protein/ribosomal protein S30EA[Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 23.97/29.22 | 7.03/6.22 | 9 | 58% | 94 | 4.00E-04 | -0.96±0.068 |
| 377 | ZP_06380559 | ORF1074 | Elongation factor TS [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 24.63/32.19 | 5.74/6.13 | 7 | 53% | 75 | 3.40E-02 | -0.83±0.076 |
| 118 | ZP_03271568 | ORF4634 | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy production and conversion | 17.70/18.27 | 5.82/6.00 | 11 | 74% | 120 | 1.00E-06 | +1.67±0.031 |
| 143 | ZP_03271568 | ORF4634 | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy production and conversion | 17.70/19.60 | 5.82/6.05 | 14 | 86% | 114 | 4.20E-06 | +0.35±0.043 |
| 70 | / | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy production and conversion | 17.70/16.05 | 5.82/4.60 | 10 | 74% | 98 | 1.70E-05 | +0.44±0.055 | |
| 517 | ABB84420 | ORF4632 | Cpci [Arthrospira platensis] | Energy production and conversion | 32.79/33.33 | 8.33/5.68 | 17 | 51% | 127 | 2.10E-07 | +0.46±0.026 |
| 47 | ZP_03271327 | ORF4103 | Allophycocyanin, beta subunit [Arthrospira maxima] | Energy production and conversion | 17.43/14.81 | 6.26/5.74 | 14 | 84% | 165 | 3.30E-11 | -0.43±0.080 |
| 185 | ZP_06383806 | ORF0728 | Hypothetical protein aplap_19246 [Arthrospira platensis] | General function prediction only | 22.95/23.52 | 4.99/5.22 | 23 | 70% | 166 | 2.60E-11 | +0.46±0.045 |
| 886 | ZP_06384147 | ORF2097 | Von Willebrand factor, type A [Arthrospira platensis] | General function prediction only | 37.19/43.74 | 5.81/5.77 | 19 | 57% | 167 | 2.10E-11 | +0.86±0.023 |
| 934 | ZP_06381540 | ORF1251 | Hypothetical protein aplap_07632 [Arthrospira platensis] | General function prediction only | 67.41/65.34 | 5.66/6.21 | 19 | 42% | 166 | 2.60E-11 | -1.63±0.019 |
| 158 | ZP_06383329 | ORF4728 | Hypothetical protein aplap_16770 [Arthrospira platensis] | Function unknown | 19.34/21.31 | 5.30/5.83 | 9 | 59% | 120 | 1.00E-06 | +0.35±0.036 |
| 78 | ZP_06384870 | ORF4030 | Hypothetical protein aplap_24737 [Arthrospira platensis] | Function unknown | 17.32/16.31 | 5.96/6.14 | 19 | 86% | 254 | 4.10E-20 | -0.39±0.098 |
| 98 | ZP_06384870 | ORF4030 | Hypothetical protein aplap_24737 [Arthrospira platensis] | Function unknown | 17.32/17.19 | 5.96/6.50 | 18 | 82% | 214 | 4.10E-16 | -0.53±0.022 |
| 339 | ZP_06384062 | ORF0361 | Peptidase S8 and S53 subtilisin kexin sedolisin [Arthrospira platensis] | Function unknown | 44.24/31.22 | 4.59/4.65 | 16 | 44% | 200 | 1.00E-14 | -0.77±0.054 |
Note: (1) In “Visible or not visible protein spots” column, “+”indicates the visible protein spots in the 45°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 45°C treatment gels.
(2) The relative abundance (Vol‰, Mean± SD) of “visible” protein calculated by the ratio of volume of each protein spot to volume of total protein spots in the whole gel.
By comparing the differentially expressed protein in the high and low temperature treatments, 5 protein spots were found to express differentially by more than 3-fold higher at 15°C than at 45°C (Figure 3). In addition, 8 protein spots were solely visible at 15°C, while 6 protein spots were solely visible at 45°C (Table 3). In total, 122 differentially expressed protein spots were obtained from the control (35°C), low (15°C) and high (45°C) temperature groups (see Table 3 A, 3B and 3C).
Figure 3. The differential protein spots between high temperature (45°C) and low temperature (15°C) treatment.
Note: The differential protein spots with >3.0-fold changes in low temperature (15°C) treatment compared to high temperature (45°C) treatment.
Table 3. The visible or not visible protein spots at 15°C vs 45°C treatment gels scanned by UNAX Powerlook 2100XL.
| Spot# | Accession NO. | ORF | Gene product | COG Functional classification | MW (kDa,Theor./ Exper.) | pI (Theor./Exper.) | Matched Peptides | Cov | Score | E-value | Visible or not visible protein spots Vol‰ (Mean± SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 516 | ZP_06381532 | ORF1242 | Cysteine synthase[Arthrospira platensis] | Amino acid transport and metabolism | 34.53/33.33 | 5.92/6.38 | 19 | 44% | 150 | 1.00E-09 | -0.58±0.022 |
| 987 | ZP_06382051 | ORF4691 | Transketolase [Arthrospira platensis] | Carbohydrate transport and metabolism | 72.98/77.27 | 5.78/5.53 | 16 | 29% | 104 | 4.20E-05 | +0.82±0.038 |
| 669 | ZP_03272326 | ORF5578 | UBA/tdIF-type NAD/FAD binding protein [Arthrospira maxima] | Coenzyme metabolism | 43.01/42.01 | 5.06/4.99 | 15 | 50% | 125 | 3.30E-07 | +0.78±0.047 |
| 256 | ZP_05256482 | ORF1829 | Radical SAM domain-containing protein [Bacteroides] | Coenzyme metabolism | 36.20/28.14 | 6.21/6.59 | 10 | 35% | 77 | 2.10E-02 | +0.49±0.035 |
| 859 | ZP_03275668 | ORF4248 | Cysteinyl-trna synthetase [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 55.01/53.51 | 5.87/6.50 | 19 | 38% | 76 | 2.80E-02 | +0.57±0.028 |
| 514 | ZP_06380559 | ORF1074 | Elongation factor TS [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 24.63/33.40 | 5.74/5.90 | 18 | 70% | 148 | 1.70E-09 | -0.33±0.027 |
| 332 | ZP_03273564 | ORF2382 | Ferredoxin [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport) | 27.02/31.29 | 5.68/5.78 | 14 | 47% | 101 | 8.30E-05 | +0.59±0.034 |
| 332* | ZP_06380716 | ORF2382 | Bidirectional hydrogenase complex protein hoxu [Arthrospira platensis] | Energy metabolism (photosynthesis, respiratory electron transport) | 27.00/31.29 | 5.54/5.78 | 17 | 68% | 143 | 5.20E-09 | +0.59±0.046 |
| 478 | ZP_06382427 | ORF2155 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy metabolism (photosynthesis, respiratory electron transport) | 29.45/32.15 | 9.25/5.85 | 25 | 70% | 206 | 2.60E-15 | -1.03±0.057 |
| 772 | ZP_06381274 | ORF0324 | UDP-glucose/GDP-mannose dehydrogenase [Arthrospira platensis] | Cell envelope biogenesis, outer membrane | 49.93/47.00 | 5.18/5.14 | 19 | 47% | 110 | 1.00E-05 | +0.50±0.050 |
| 941 | ZP_03275087 | ORF0324 | Nucleotide sugar dehydrogenase [Arthrospira maxima] | Cell envelope biogenesis, outer membrane | 34.72/47.54 | 5.26/5.16 | 30 | 72% | 249 | 1.30E-19 | -1.02±0.086 |
| 118 | ZP_06382587 | Hypothetical protein aplap_12998 [Arthrospira platensis] | Function unknown | 19.30/17.10 | 9.47/6.55 | 15 | 74% | 183 | 5.20E-13 | +0.66±0.033 | |
| 183 | ZP_03274017 | ORF1894 | Uncharacterized protein/domain associated with gtpase-like protein [Arthrospira maxima] | Function unknown | 20.09/23.37 | 5.74/5.93 | 5 | 32% | 78 | 1.80E-02 | -0.50±0.049 |
| 425 | ZP_06384062 | ORF0361 | Peptidase S8 and S53 subtilisin kexin sedolisin [Arthrospira platensis] | Function unknown | 44.24/30.81 | 4.59/4.46 | 24 | 54% | 156 | 2.60E-10 | -0.56±0.021 |
Note: (1) In “Visible or not visible protein spots” column, “+”indicates the solely visible protein spots in the 15°C treatment gels, and “-”indicates the solely visible protein in the 45°C treatment gels.
(2) The relative abundance (Vol‰, Mean± SD) of “visible” protein calculated by the ratio of volume of each protein spot to volume of total protein spots in the whole gel.
PMF of the differentially expressed protein spots and MALDI-TOF MS analysis
The TwoClassDif (RVM-T test) method with a cutoff of p-value <0.05 and FDR <0.05 was applied to analyze the three different temperature treatments. Only those with reproducibility and the fold change with >3.0-fold threshold were considered to be differentially expressed protein spots. The confidence in the peptide mass fingerprinting matches (p<0.05) was based on the MOWSE Score more than 75. The mass spectrum of identified subset protein spots is shown in Figure S2. Using MASCOT searching, 122 positively expressed protein spots were categorized into several groups according to their functions, of which 116 proteins were demonstrated to have high homology with the Arthrospira platensis genus. However, the other 6 proteins (YP_002248469, YP_002485086, ZP_04040440, YP_001999788, YP_912068, ZP_05256482) had high homology with other prokaryotes, which belonged to Hermodesulfovibrio yellowstonii, Cyanothece sp. PCC 7425, Meiothermus ruber DSM 1279, Mycoplasma arthritidis, Chlorobium phaeobacteroides and Bacteroides, respectively. All of the above prokaryotes are high temperature-tolerant bacteria with the exception of Cyanothece sp. PCC 7425 (see Tables S3A, S3B and S3C).
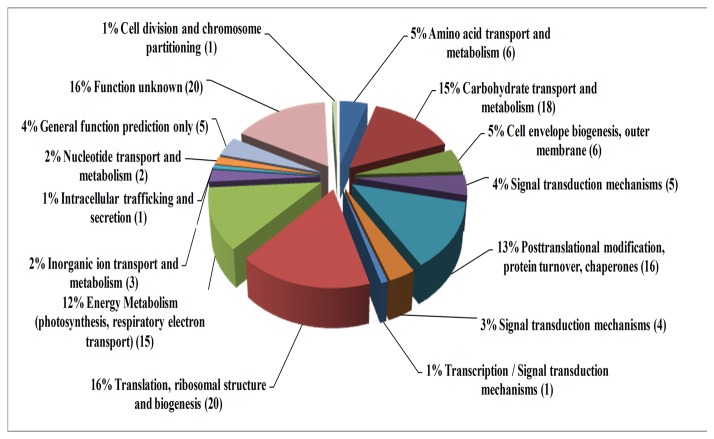
By comparing the theoretical and actual values of Mw and pI of Matched Proteins acquired by MS and Gel Map Analytical Software, we performed COG function prediction and classified the 122 positively expressed proteins into 15 functional categories. All kinds of functional protein spots differentially expressed at different temperatures as summarized in Table 4, and the proportion of the different kinds of functional protein is shown in Figure 4 on the basis of proven high credibility. At 15°C, the highest proportion of gene functions was related to energy metabolism (photosynthesis, respiratory and electron transport), carbohydrate transport and metabolism, post-translational modification, protein turnover, chaperones and coenzyme metabolism; in addition there were some unknown function genes. Similarly at 45°C, the highest proportion of gene functions was concerned with energy metabolism (photosynthesis, respiratory and electron transport), while some protein functions were related to carbohydrate transport, metabolism and translation, ribosomal structure and biogenesis. In summary, a large portion of the differentially expressed protein spots that responded to high or low temperatures were related to phycobilisome (PBS), which could affect algal photosynthesis and energy metabolism.
Table 4. Functional classification of the differential protein spots in different temperature treatments.
| NO. | COG classification | Total number at 15°C | Total number at 35°C | Total number at 45°C | Total number |
|---|---|---|---|---|---|
| 1 | Amino acid transport and metabolism | 3 | 1 | 2 | 6 |
| 2 | Carbohydrate transport and metabolism | 7 | 7 | 4 | 18 |
| 3 | Cell envelope biogenesis, outer membrane | 4 | 1 | 1 | 6 |
| 4 | Coenzyme metabolism | 5 | / | / | 5 |
| 5 | Post-translational modification, protein turnover, chaperones | 7 | 7 | / | 14 |
| 6 | Signal transduction mechanisms | 3 | 1 | / | 4 |
| 7 | Transcription / Signal transduction mechanisms | 1 | / | / | 1 |
| 8 | Translation, ribosomal structure and biogenesis | 3 | 10 | 3 | 16 |
| 9 | Energy Metabolism (photosynthesis, respiratory electron transport) | 8 | 6 | 7 | 21 |
| 10 | Inorganic ion transport and metabolism | 2 | / | 1 | 3 |
| 11 | Intracellular trafficking and secretion | 1 | / | / | 1 |
| 12 | Nucleotide transport and metabolism | 1 | 1 | / | 2 |
| 13 | General function prediction only | 1 | 1 | 2 | 4 |
| 14 | Function unknown | 9 | 5 | 6 | 20 |
| 15 | Cell division and chromosome partitioning | / | 1 | / | 1 |
| 16 | Total function proteins | 54 | 42 | 26 | 122 |
Figure 4. COG functional classification of 122 positively differential expression proteins.
qRT-PCR analyses of the differentially expressed genes
The purity and concentration of total RNA
Based on agarose gel electrophoresis determination, the extracted RNA in the low (15°C), control (35°C) and high (45°C) temperature treatments showed good integrity and high purity, and had no degradation (see Figure S3). The OD260/OD280 triplicate values were 2.09, 2.03 and 2.04, and the OD260/230 triplicate values were 2.08, 2.24 and 2.19 for the low (15°C), control (35°C) and high (45°C) temperature groups, respectively. The RNA concentrations were 241.2, 213.6 and 209.5 ng/µL in the low (15°C), control (35°C) and high (45°C) temperature treatments, respectively. Both OD260/OD280 and OD260/230 values were greater than 2.0, demonstrating no contamination of RNA by proteins, salts or carbohydrates.
Standard curve for qRT-PCR
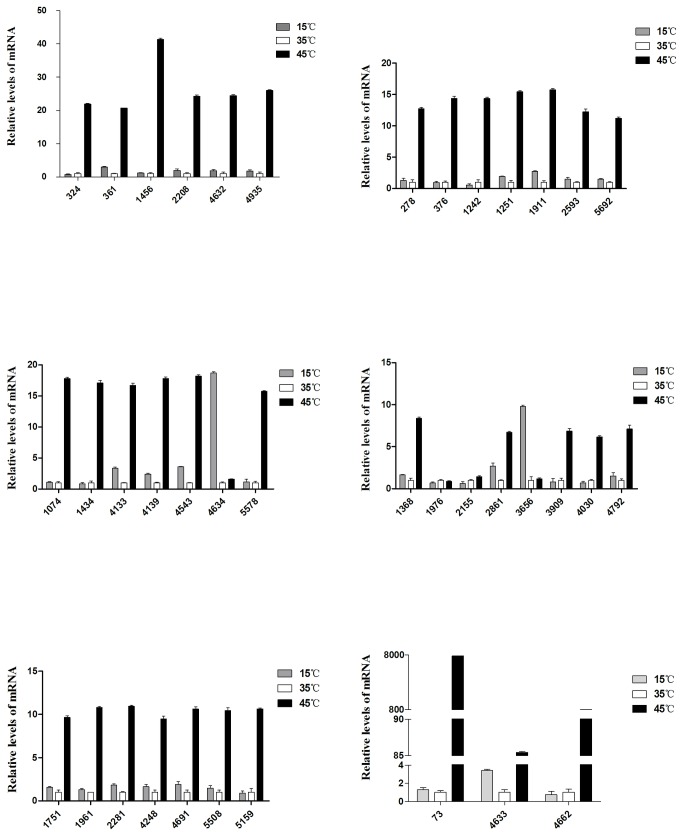
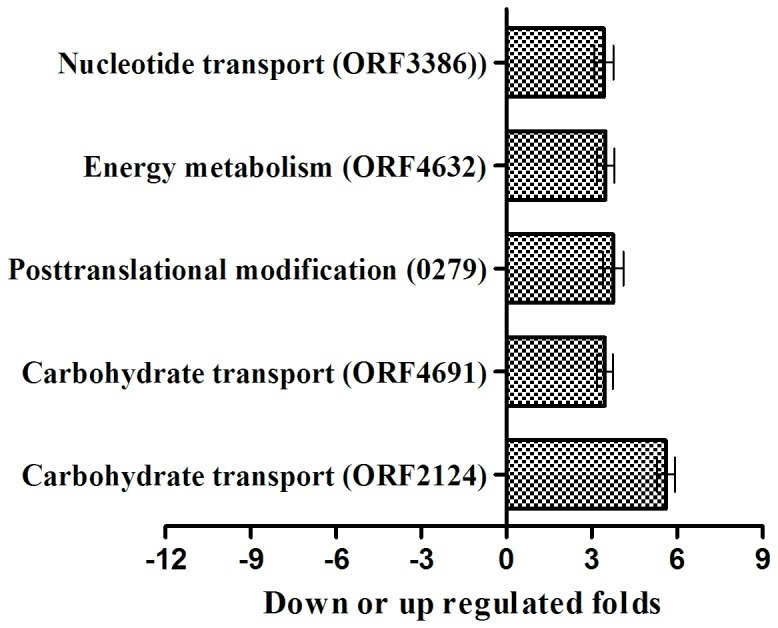
We selected a total of 38 genes for further validation, of which 23 genes were found to be significantly different between the 15°C treatment and control (35°C), 13 genes between the 45°C treatment and control, and 2 genes between the 15 and 45°C treatments (ORF4248 up-regulated at 15°C and ORF0324 down-regulated at 45°C). The information for annotation and metabolic pathway of the 38 genes is summarized in Table 5, and the consistency between their transcription level and gene expression was confirmed by qRT-PCR. All of the melting curves showed a single peak in the tested range of standard curves, suggesting good specificity (standard curves not shown in Figure). A non-detectable fluorescence signal in negative and blank controls indicated that the reaction system was not contaminated during the experimental procedures. The calibration curves for 16S rRNA as endogenous control and target genes, regression coefficients and amplification efficiencies were between 98.2-99.8% and 81.1-117.2%, which indicated efficient amplification with high specificity (see Table S2).
Table 5. Annotation and proposed metabolic pathways of the 38 differential protein spots obtained from the MS-searching.
| Spot | ORF | Accession NO. | Gene Product | COG Functional classification | MW (kDa,Theor./ Exper.) | pI (Theor./Exper.) | Matched Peptides | Cov | MASCT Score | E-value | Fold change Mean± SD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 859 | ORF4248 | ZP_03275668 | Cysteinyl-trna synthetase [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 55.01/53.51 | 5.87/6.50 | 19 | 38% | 76 | 2.80E-02 | 6.36±0.41 |
| 433 | ORF1074 | ZP_06380559 | Elongation factor TS [Arthrospira platensis] | Translation, ribosomal structure and biogenesis | 24.63/31.08 | 5.74/5.90 | 21 | 77% | 155 | 3.30E-10 | / |
| 224 | ORF0073 | ZP_03275641 | Ribosomal protein L6 [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 19.69/25.13 | 10.25/5.63 | 11 | 40% | 104 | 4.20E-05 | / |
| 283 | ORF4662 | ZP_03271593 | Sigma 54 modulation protein/ribosomal protein S30EA [Arthrospira maxima] | Translation, ribosomal structure and biogenesis | 23.97/29.22 | 7.03/6.22 | 9 | 58% | 94 | 4.00E-04 | / |
| 517 | ORF4632 | ABB84420 | Cpci [Arthrospira platensis] | Energy production and conversion | 32.79/33.33 | 8.33/5.68 | 17 | 51% | 127 | 2.10E-07 | / |
| 143 | ORF4634 | ZP_03271568 | Phycocyanin, alpha subunit [Arthrospira maxima] | Energy production and conversion | 17.70/19.60 | 5.82/6.05 | 14 | 86% | 114 | 4.20E-06 | / |
| 564 | ORF4633 | ZP_06380687 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy production and conversion | 30.87/34.48 | 7.82/6.60 | 30 | 71% | 338 | 1.70E-28 | 3.09±0.28 |
| 486 | ORF2155 | ZP_06382427 | Phycobilisome linker polypeptide [Arthrospira platensis] | Energy production and conversion | 29.45/32.42 | 9.25/6.25 | 25 | 70% | 237 | 2.10E-18 | 5.39±0.35 |
| 941 | ORF0324 | ZP_03275087 | Nucleotide sugar dehydrogenase [Arthrospira maxima] | Cell envelope biogenesis, outer membrane | 34.72/47.54 | 5.26/5.16 | 30 | 72% | 249 | 1.30E-19 | / |
| 868 | ORF5692 | ZP_06383693 | Phosphateuridyltransferase/glucosamine phosphateacetyl transferase[Arthrospira platensis] | Cell envelope biogenesis, outer membrane | 50.14/54.88 | 5.74/6.18 | 23 | 50% | 235 | 3.30E-18 | 3.38±0.27 |
| 213 | ORF1751 | ZP_06381424 | NAD-dependent epimerase/dehydratase [Arthrospira platensis] | Cell envelope biogenesis, outer membrane | 23.63/26.06 | 5.15/5.49 | 23 | 78% | 231 | 8.30E-18 | 3.19±0.28 |
| 160 | ORF4935 | ZP_06381012 | Acetolactate synthase regulatory | Amino acid transport and metabolism | 19.26/22.13 | 6.62/6.80 | 14 | 64% | 154 | 4.20E-10 | 3.10±0.22 |
| 535 | ORF3909 | ZP_06382239 | Aspartate-semialdehyde dehydrogenase [Arthrospira platensis] | Amino acid transport and metabolism | 37.27/38.57 | 5.38/5.72 | 22 | 64% | 209 | 1.30E-15 | 3.43±0.29 |
| 492 | ORF1242 | ZP_06381042 | Cysteine synthase A [Arthrospira platensis] | Amino acid transport and metabolism | 34.84/37.37 | 5.33/5.85 | 23 | 67% | 151 | 8.30E-10 | 3.39±0.15 |
| 648 | ORF0278 | ZP_03273305 | Pyridoxal-5'-phosphate-dependent protein beta subunit [Arthrospira maxima] | Amino acid transport and metabolism | 34.84/37.95 | 5.28/5.23 | 26 | 69% | 241 | 8.30E-19 | / |
| 664 | ORF5578 | ZP_03272326 | UBA/THIF-type NAD/FAD binding protein [Arthrospira maxima] | Coenzyme metabolism | 43.01/41.67 | 5.06/5.20 | 15 | 49% | 147 | 2.10E-09 | 3.75±0.28 |
| 235 | ORF4133 | ZP_03275242 | Pyridoxal phosphate biosynthetic protein pdxj [Arthrospira maxima] | Coenzyme metabolism | 26.10/27.02 | 5.30/5.52 | 7 | 47% | 114 | 4.20E-06 | 4.69±0.43 |
| 536 | ORF4139 | ZP_06381750 | Tetrahydrofolatede Hydrogenase/ cyclohydrolase [Arthrospira platensis] | Coenzyme metabolism | 30.92/38.66 | 6.01/6.79 | 10 | 32% | 76 | 2.50E-02 | 5.49±0.42 |
| 747 | ORF5508 | ZP_03272404 | Ribulose-bisphosphate carboxylase[Arthrospira maxima] | Carbohydrate transport and metabolism | 53.91/46.33 | 6.04/5.78 | 25 | 42% | 230 | 1.00E-17 | / |
| 1120 | ORF0376 | ZP_06381999 | Transketolase domain protein [Arthrospira platensis] | Carbohydrate transport and metabolism | 69.60/64.11 | 5.19/5.52 | 41 | 64% | 297 | 2.10E-24 | / |
| 928 | ORF4691 | ZP_06382051 | Transketolase [Arthrospira platensis] | Carbohydrate transport and metabolism | 72.98/64.76 | 5.78/5.75 | 21 | 45% | 204 | 4.10E-15 | / |
| 648 | ORF3656 | ZP_06380758 | Fructose-1,6-bisphosphate Aldolase [Arthrospira platensis] | Carbohydrate transport and metabolism | 38.99/41.19 | 5.60/4.49 | 13 | 33% | 168 | 1.60E-11 | / |
| 507* | ORF4543 | ZP_06383180 | Hypothetical protein aplap15993 [Arthrospira platensis] | Cell division and chromosome partitioning | 38.08/38.00 | 4.59/4.65 | 21 | 58% | 190 | 1.00E-13 | -5.07±0.43 |
| 156 | ORF1368 | ZP_03273475 | Redoxin domain protein[Arthrospira maxima] | Post-translational modification, protein turnover, chaperones | 19.78/21.51 | 4.88/5.18 | 15 | 66% | 133 | 5.20E-08 | 5.85±0.43 |
| 916 | ORF1976 | ZP_03275215 | Chaperonin GroEL [Arthrospira maxima] | Post-translational modification, protein turnover, chaperones | 58.21/57.65 | 5.00/5.25 | 28 | 56% | 183 | 5.20E-13 | 3.84±0.29 |
| 275 | ORF2281 | ZP_06380867 | Peptidyl-prolyl cis-trans isomerase, cyclophilin type [Arthrospira platensis] | Post-translational modification, protein turnover, chaperones | 23.92/28.93 | 4.74/4.49 | 6 | 36% | 75 | 3.60E-02 | / |
| 805 | ORF1434 | ZP_06380920 | ChaperoninGroEL[Arthrospira platensis] | Post-translational modification, protein turnover, chaperones | 57.43/50.62 | 5.00/4.47 | 24 | 50% | 271 | 8.20E-22 | / |
| 210 | ORF2861 | ZP_06382415 | Stress protein [Arthrospira platensis] | Signal transduction mechanisms | 22.20/26.12 | 4.93/5.23 | 14 | 78% | 111 | 8.30E-06 | 3.82±0.19 |
| 170 | ORF4792 | ZP_06382815 | DNA starvation/stationary phase protection protein Dps [Arthrospira platensis] | Inorganic ion transport and metabolism | 19.67/23.83 | 4.93/5.04 | 13 | 70% | 110 | 8.30E-06 | 3.07±0.27 |
| 165 | ORF1911 | ZP_06383116 | Adenylylsulfate kinase [Arthrospira platensis] | Inorganic ion transport and metabolism | 19.90/21.51 | 5.22/5.18 | 12 | 66% | 149 | 1.30E-09 | 3.32±0.22 |
| 934 | ORF1251 | ZP_06381540 | Hypothetical protein aplap_07632 [Arthrospira platensis] | General function prediction only | 67.41/65.34 | 5.66/6.21 | 19 | 42% | 166 | 2.60E-11 | / |
| 137 | ORF5159 | ZP_03271326 | Phycobilisome protein [Arthrospira maxima] | Energy metabolism (photosynthesis, respiratory electron transport | 17.44/19.04 | 4.89/4.93 | 16 | 62% | 148 | 1.70E-09 | / |
| 961 | ORF2208 | ZP_06383545 | Hypothetical protein aplap_17874 [Arthrospira platensis] | Function unknown | 18.76/65.83 | 5.42/5.58 | 13 | 77% | 94 | 4.30E-04 | 4.10±0.28 |
| 413 | ORF0361 | ZP_06384062 | Peptidase S8 and S53 subtilisin kexin sedolisin [Arthrospira platensis] | Function unknown | 44.24/34.26 | 4.59/4.57 | 22 | 49% | 166 | 2.60E-11 | 5.25±0.48 |
| 275 | ORF1961 | ZP_06380972 | Ppic-type peptidyl-prolyl cis-trans isomerase | Function unknown | 31.57/28.94 | 5.27/4.94 | 22 | 74% | 190 | 1.00E-13 | 3.12±0.24 |
| 216 | ORF1456 | ZP_06380822 | Pentapeptide repeat-containing protein [Arthrospira platensis] | Function unknown | 19.86/24.68 | 5.13/5.44 | 16 | 58% | 125 | 3.30E-07 | 3.32±0.29 |
| 680 | ORF4030 | ZP_06384870 | Hypothetical protein aplap_24737 [Arthrospira platensis] | Function unknown | 17.32/15.87 | 5.96/6.23 | 20 | 93% | 224 | 4.20E-17 | 3.19±0.21 |
| 188 | ORF2593 | ZP_03274149 | Beta-propeller repeat TECPR[Arthrospira maxima] | Function unknown | 22.58/25.41 | 4.81/4.72 | 9 | 34% | 89 | 1.30E-03 | / |
Note: (1) Fold change indicates Mean± SD; (2) “/ ” indicates the visible or not visible protein spots scanned by UNAX Powerlook 2100X
The relative transcription level of genes using qRT-PCR
The mRNA relative transcription levels of positively expressed proteins detected by qRT-PCR for the control, high and low temperature treatments are shown in Figure 5. At 15°C, there were 23 genes with >3-fold differential expression, among which 16 genes were up-regulated and 7 genes were down-regulated on the protein level. However, the transcription levels of 17 genes were up-regulated, while those of 6 genes were down-regulated. Of the 17 up-regulated genes, the genes with >2-fold transcription level were ORF3656, ORF4133, ORF2861, ORF4139 and ORF361. The 6 down-regulated genes all exceeded a one-fold change. At 45°C, the transcription levels of 10 genes were up-regulated more than 3-fold, while those of 3 genes (ORF73, ORF4543 and ORF1251) were down-regulated in 13 differentially expressed proteins. At the transcription level, 10 genes were up-regulated more than 3-fold, while ORF4634 and ORF2155 displayed less than a 2-fold change. When comparing low with high temperature treatments, the transcription and expression levels of ORF324 were consistent, but those of ORF4248 were inconsistent, i.e., the transcription of ORF4248 was down-regulated and the translation was up-regulated. More interestingly, the fold changes of ORF4633 and ORF73 with up-regulated transcription levels were more than 85-fold and 7867-fold, respectively. Of the 38 target genes, the protein expression of 26 genes was consistent with gene transcription, representing 68.4% of the total target genes. The remaining 12 genes showed inconsistent protein expression with transcription level, representing 31.6% of the total target genes. A comparison of transcription and protein expression of the target genes is summarized in Table 6.
Figure 5. The differential expression of the selected 38 genes for the control and the temperature-stress treatment group by qRT-PCR.
Table 6. The comparison between qPCR and proteomic analysis result.
| Accession NO. | ORF | qPCR results 15-2 -⊿⊿Ct /35-2 -⊿⊿Ct | Fold change from proteomic results | Consistency |
|---|---|---|---|---|
| ZP_06381424 | 1751 | 22.9/14.6 ↑ | ↑ 3.19 | Y |
| ZP_06380972 | 1961 | 14.0/10.6 ↑ | ↑ 3.12 | Y |
| ZP_06382815 | 4792 | 50.6/33.2 ↑ | ↑ 3.07 | Y |
| ZP_06383693 | 5692 | 30.4/20.3 ↑ | ↑ 3.38 | Y |
| ZP_06383545 | 2208 | 1.97/1.00 ↑ | ↑ 4.1 | Y |
| ZP_03275242 | 4133 | 0.37/0.11 ↑ | ↑ 4.69 | Y |
| ZP_06381012 | 4935 | 0.60/0.34 ↑ | ↑ 3.1 | Y |
| ZP_03272326 | 5578 | 5.30e-05/4.59e-05 ↑ | ↑ 3.75 | Y |
| ZP_06380920 | 1434 | 0.87/1.00 ↓ | ↓ - | Y |
| ZP_06382415 | 2861 | 11.1/4.09 ↑ | ↑ 3.82 | Y |
| ZP_06381750 | 4139 | 2.24/0.94 ↑ | ↑ 5.49 | Y |
| ZP_03271593 | 4662 | 2.38e-05/3.26e-05 ↓ | ↓ - | Y |
| ZP_03273475 | 1368 | 5.78/3.43 ↑ | ↑ 3.45 | Y |
| ZP_06384062 | 0361 | 1.46/0.50 ↑ | ↑ 5.25 | Y |
| ZP_06381042 | 1242 | 0.56/1.00 ↓ | ↑ 3.39 | N |
| ZP_06380867 | 2281 | 25.1/13.6 ↑ | ↓ -3.12 | N |
| ZP_06382239 | 3909 | 4.41/5.42 ↓ | ↑ 3.4 | N |
| ZP_03275215 | 1976 | 0.56/1.35 ↓ | ↑ 3.84 | N |
| ZP_06380758 | 3656 | 3.02/0.31 ↑ | ↓ -4.39 | N |
| ZP_03271326 | 5159 | 2.51/2.77 ↓ | ↑ + | N |
| ZP_03274149 | 2593 | 2.88/1.90 ↑ | ↓ + | N |
| ZP_06382051 | 4691 | 2.55/1.33 ↑ | ↓ + | N |
| ZP_03272404 | 5508 | 25.5/17.3 ↑ | ↓ + | N |
| ORF | qPCR results 45-2- ⊿⊿ Ct/35-2 -⊿⊿Ct | Fold change from proteomic results | Consistency | |
| ZP_03275641 | 0073 | 0.80/0.0001 ↑ | ↑ + | Y |
| ZP_06383116 | 1911 | 25.3/1.61 ↑ | ↑ 3.32 | Y |
| ABB84420 | 4632 | 0.50/0.02 ↑ | ↑ + | Y |
| ZP_03271568 | 4634 | 0.41/0.26 ↑ | ↑ + | Y |
| ZP_06380822 | 1456 | 14.0/0.34 ↑ | ↑ 3.32 | Y |
| ZP_06382427 | 2155 | 0.17/0.12 ↑ | ↑ 5.39 | Y |
| ZP_03273305 | 0278 | 108/8.49 ↑ | ↑ + | Y |
| ZP_06381999 | 0376 | 14.7/1.02 ↑ | ↑ + | Y |
| ZP_0638487 | 4030 | 193/31.2 ↑ | ↑ 3.19 | Y |
| ZP_06380687 | 4633 | 5.58/0.07 ↑ | ↑ 3.09 | Y |
| ZP_06380559 | 1074 | 17.8/1.00 ↑ | ↑ + | Y |
| ZP_06383180 | 4543 | 19.6/1.08 ↑ | ↓ -5.07 | N |
| ZP_06381540 | 1251 | 15.5/1.00 ↑ | ↑ - | N |
| ORF | qPCR results 45-2-⊿⊿Ct/15-2-⊿⊿Ct | Fold change from proteomic results | Consistency | |
| ZP_03275087 | 0324 | 9.5/0.32 ↑ | ↑ + | Y |
| ZP_03275668 | 4248 | 36.2/6.2 ↑ | ↓ -6.36 | N |
Note: (1) “Y” indicates the transcriptional level and expression of gene is consistency, “N” indicates inconsistency
(2) In “Fold change” column, “+” or “-” indicates the visible or not visible protein spots.
(3) The positive and negative fold indicate the protein spots with >3-fold up-regulation and down-regulation in 15°C and 45°C treatment group vs 35°C.
Finally, we performed metabolic pathway positioning using KEGG analysis, and found 27 genes with >3-fold up-regulated proteins and 5 genes with >3-fold down-regulated proteins. In addition, 18 protein spots were only observed in the 15°C treatment, while they were not visible in the control group. As shown in Figure 1 and Table 1, these genes were involved in energy metabolism (photosynthesis, respiratory electron transport), post-translational modification, protein turnover, chaperones, carbohydrate transport and metabolism, coenzyme metabolism and cell envelope biogenesis. At 45°C, there were 10 genes with >3-fold up-regulated proteins and 2 genes with >3-fold down-regulated proteins. A total of 12 protein spots were only visible in the 45°C treatment and not visible in the control group. These genes were involved in energy metabolism, carbohydrate transport and translation. Moreover, 7 genes for the 15°C treatment and 8 genes for the 45°C treatment were found to have unknown functions, belonging to hypothetical or uncharacterized proteins.
Discussion
Sample preparation
In proteome studies, sample preparation is the first key step especially for algal cell samples, which contain many secondary metabolites that may interfere with electrophoresis profiles. 2-DE procedures were optimized using three approaches. Firstly, the proteins were sedimented by TCA/cold acetone, which inhibited protein hydrolysis due to the presence of protease and removed a large proportion of secondary metabolites, such as pigments, quinones and phenols. Secondly, we chose pH 4-7 IPG drystrips, with a narrow pH range, instead of pH 3-10 drystrips, which improved the resolution of protein spots in 2-DE profiles by increasing the loading quantity of the sample and the numbers of protein spots with low abundance. Finally, we utilized the improved protein dissolving buffer to enhance the dissolving efficiency for proteins.
If biological questions can be answered using quantitative proteomics, it is essential to design experiments which have sufficient power to be able to detect changes in expression. Sample pooling is a strategy that can be used to reduce the variance but still allow studies to encompass biological variation. Underlying sample pooling strategy is the biological averaging assumption that the measurements taken on the pool are equal to the average of the measurements taken on the individuals. Karp et al. reported no evidence of a systematic bias triggered by sample pooling for DIGE and that pooling can be useful in reducing biological variation [26,27]. The previous study also demonstrated an alternative smart pooling strategy in which different samples, not necessarily biological replicates, are pooled in an information theoretic efficient way [28]. Pooling of RNA samples is generally applied to obtain samples that represent the average signal of biological replicates of a single treatment. For toxicogenomics, pooling RNA of samples treated by different compounds could in the same way summarize these compounds to a single sample with average signals per class. Pooling RNA from compounds of a class substantially increased power to detect significantly regulated genes between classes because variability between pooled samples was much lower. Within pools the vast majority of genes maintained patterns of expression compared to the separately hybridized samples, especially in regulated genes [29]. Engel et al. found that the most abundant T-RFLP (terminal restriction fragment polymorphism) peaks were generally shared between biological replicates and pooled samples, and that sample pooling was still effective for the analyses of ecological key players [30]. Especially for homogeneous biological samples, pooling of RNA samples of biological replicates can be generally applied for representing the average information of biological replicates of a single treatment [29]. Therefore, we mixed the extracted protein samples in the three biological replicates into one sample for 2-DE in order to simplify the subsequent 2-DE procedures considering that Arthrospira (Spirulina) platensis was a kind of homogeneous biological material.
Target genes and its related mechanisms
Temperature is an important abiotic factor restricting the growth and distribution of cyanobacteria. The up- or down-regulation of the target genes, as a response to adverse environmental conditions, can lead to the corresponding regulation of their encoded proteins. Thus, we investigated the differentially expressed proteins of ASP at different temperatures by proteomic techniques and identified differentially expressed genes. It can be expected that some differentially expressed genes play an important role in response to abnormal temperatures.
Some findings suggested that temperature stress for a long time below the freezing point could result in destroyed cell structure, cell cycle current stoppage, solution leakage from cells, and a broken equilibrium state with the inner hormone system. Therefore, the cyanobacteria will experience a series of metabolic obstacles [31]. ASP can tolerate low temperature by increasing the content of intracellular soluble sugar, protein, and free proline to change the metabolism in vivo [32]. The effects of high temperature on ASP mainly include direct and indirect damage associated with photosynthesis and respiration metabolic activity, inhibiting biological activity of large molecules, and accumulating toxic substances that lead to oxidative damage of biological macromolecules [33].
(1): Heat shock proteins (protein turnover and chaperones)
We found that the seven genes related to protein chaperones polypetide folding and protein turnover in ASP were significantly regulated or newly produced under temperature stresses (Table S3). The Chaperonin Hsp60/GroEL (ORF1976 and ORF1434) is a special protein associated with nascent polypeptide chain folding, oligomeric protein assembly and transmembrane transport [34]. The Hsp60 in mitochondrion mainly participate in protein processing, transport, positioning and assembly. The chloroplast Hsp60 encoded by nuclear genes abundantly exists under normal conditions. However as a response to high temperature, the denatured proteins increase in vivo, and Hsp60 binds to the denatured protein to maintain their soluble state. In the presence of Mg2+-ATP, Hsp60 can change folding proteins to refold into the active conformation, or degrade the denatured protein [35]. For example, Hsp60 can refold the Rubisco enzyme of cyanobacteria and malate dehydrogenase of mitochondrion in vitro [36]. This type of molecular chaperone usually plays an important role in protein migration, translation mechanisms on thylakoid membrane surfaces and improving the fluidity of membranes in order to maintain metabolic activity at low temperatures [22,37]. The up-regulation of the molecular chaperone, Hsp60/GroEL, was also found on exposure to low temperature. We inferred that Hsp60/GroEL was involved in biochemical mechanisms through cells that sense temperature changes and protect ASP from being damaged in response to low temperature.
Moreover, the functional identification results demonstrated that ORF2155 (ZP_06382427), ORF4632 (ZP_06380688) and ORF4633 (ZP_06380637) were related to phycobilisome linker polypeptides, and their significant changes were regulated by Hsp70 and Hsp40 under high or low temperatures. Both Hsp70 and Hsp40 chaperone families in the cytoplasm or the ER play a critical role in folding and secretion of heterologous proteins. The Hsp70 proteins transiently bind and release polypeptides in an ATP-dependent cycle and the Hsp40 proteins regulate this cycle by modulating the ATPase activity of Hsp70. These chaperones direct nascent polypeptide chains through a series of specialized compartments within the secretory pathway. Only those proteins that have surpassed the proofreading and quality control check points in the pathway are secreted. The partner proteins bind to nascent peptide chains thereby stabilizing and directing them for translocation into the ER [38].
(2): Energy metabolism (photosynthesis, respiratory and electron transport)
As summarized in Tables 7 and 8, the expression levels of some genes involved in energy metabolism (photosynthesis, respiratory and electron transport) were significantly changed. For example, Ferredoxin (Fd, ORF2382) was expressed at low temperature, while this was not the case at high temperature.
Table 7. The process of metabolism-related differential protein spots at 15°C.
| 15°CSpots# | ORF | Accession NO. | Energy metabolism | Fold change | Name |
|---|---|---|---|---|---|
| 137 | ORF5159 | ZP_03271326 | Allophycobilisome, alpha subunit | + | ApcA |
| 140 | ORF4634 | ZP_03271568 | Phycocyanin, alpha subunit | + | CpcA |
| 147 | ORF4634 | ZP_03271568 | Phycocyanin, alpha subunit | + | CpcA |
| 332 | ORF2382 | ZP_03273564 | Ferredoxin | + | / |
| 332 | ORF2382 | ZP_06380716 | Bidirectional hydrogenase complex protein HoxU | + | / |
| 435 | ORF4632 | ZP_06380688 | Phycocyanin-associated rod linker polypeptide | 3.26± 0.29 | CpcC |
| 436 | ORF4632 | ZP_06380688 | Phycocyanin-associated rod linker polypeptide | 4.17± 0.28 | CpcC |
| 390 | ORF2155 | ZP_06382427 | Phycobilisome rod-core linker polypeptide | + | CpcG |
Note: In “Fold change” column, “+”indicates the solely visible protein spots in 15°C treatment gels.
The positive fold indicates the protein spots with >3-fold up-regulation in 15°C vs 35°C treatment group.
Table 8. The process of metabolism-related differential protein spots at 45°C.
| 45°C Spots# | ORF | Accession NO. | Energy metabolism | Fold change | Name |
|---|---|---|---|---|---|
| 118 | ORF4634 | ZP_03271568 | Phycocyanin, alpha subunit | + | CpcA |
| 143 | ORF4634 | ZP_03271568 | Phycocyanin, alpha subunit | + | CpcA |
| 70 | Phycocyanin, alpha subunit | + | CpcA | ||
| 564 | ORF4633 | ZP_06380687 | Phycocyanin-associated rod linker polypeptide | 3.09± 0.28 | CpcC |
| 478 | ORF2155 | ZP_06382427 | Phycobilisome rod-core linker polypeptide | + | CpcG |
| 486 | ORF2155 | ZP_06382427 | Phycobilisome rod-core linker polypeptide | 5.39± 0.35 | CpcG |
Note: In “Fold change” column, “+”indicates the solely visible protein spots in 45°C treatment gels.
The positive fold indicates the protein spots with >3-fold up-regulation in 45°C vs 35°C treatment group.
In the process of photosynthesis, [2Fe-2S]-Fd accepts electrons from the PSI and transfers them to Fd:NADP+ oxidoreductase to reduce the NADP+ [39], suggesting that Fd plays an important role in PSI. However, Fd (protein spot 332, ORF2382) was suppressed at high temperature, which could result in the significant inhibition of PSI at high temperatures [40].
In addition, a large portion of the differentially expressed protein spots was related to phycobilisome (PBS). PBS is a pigment and protein complex that attaches to the outer surface of thylakoid membranes [41,42]. After the differentially expressed protein spots were characterized with their associated metabolic pathway with KEGG functional annotation, we found four photosynthesis-related proteins ApcA (ORF5159), CpcA (ORF4634), CpcC (ORF4632) and CpcG (ORF2155) at 15°C, and three photosynthesis-related proteins CpcA (ORF4634), CpcC (ORF4633) and CpcG (ORF2155) at 45°C. ApcA (ORF5159) is a part of the allophycocyanin (APC) complex belonging to the core of phycobilisome, CpcA (ORF4634) is an alpha subunit of phycocyanin (PC), and CpcC (ORF4632, ORF4633) and CpcG (ORF2155) are phycobilisome linker polypeptides [42].
It is worthy to note that the expression level of CpcC and CpcG increased by more than 3-fold (especially for CpcG by 5.4-fold at 45°C) when ASP was subjected to low or high temperatures (15 or 45°C). The increase of phycobilisome linker polypeptide CpcC and CpcG made the linker more stable between phycocyanin and allo-phycocyanin. These phenomena would protect phycobilisome from uncoupling under abnormal temperatures. Additionally, the expression level of phycobilisome protein ApcA and the phycocyanin alpha subunit CpcA increased at 15°C, and the level of CpcA increased at 45°C [43,44].
High temperature has a remarkable effect on multiple target sites of thylakoid membranes, which can further affect electronic transfers associated with photosynthesis [41]. The decreasing photochemical activity of PSII results from oxygen-evolving complex inactivation of PSII, structural changes in the thylakoid membranes [45], decreasing of the PSII-mediated electronic transfer rate, and changes of the antenna system conformation by high temperature. On the other hand, cyanobacteria increase the separation between the PBS supermolecules and the thylakoid membrane, which further prompts the disintegration of PBS supermolecules [46]. The expression level of linking polypeptides CpcC and CpcG significantly increased when compared to the control, which demonstrated that ASP took control measures to prevent phycobilisomes from dissociation due to high or low temperatures. Increasing the expression of CpcC and CpcG would lead to increasing stability of phycocyanin and the related stability between PC and APC. In addition, a slight increase of expression level for ApcA and CpcA subunits was observed at 15°C, and a similar case for CpcA subunits was observed at 45°C. The above results imply adaptation mechanisms of ASP to temperature, which helps explain ASP’s survival and growth under abnormal temperatures [47].
Phycobiliproteins are highly variable in abundance under different environmental conditions and very dynamic in their synthesis and turnover or group modification. For most phycobiliproteins, post-translational modifications are catalyzed by enzymes called bilin lyases, and the methylation of a conserved asparagine (Asn) present at beta-72, which occurs on the beta-subunits of all phycobiliproteins [48].These core proteins, such as ApcA, CpcA and CpcG, are active in light harvesting and can not be completely absent from the control (35°C) group. Our experimental results were acquired by screening the differentially expressed proteins according to relevant statistical standards. Perhaps, due to the high variability of phycobiliproteins, they all showed a significantly expressed up-regulation at 45°C and 15°C. On the contrary, the expression of phycobiliproteins at the control temperature (35°C) was so low that they did not fall within the selected statistical criteria range, and thus they were considered as negatively expressed proteins. As for ApcA, CpcA and CpcG, whether or not their location on the 2DE gels changes in the different temperature treatments remains a question for further investigation. Although some of the proteins were not found to be positively expressed, this does not necessarily demonstrate the non-existence of these proteins in the gels.
(3): Carbohydrate transport and metabolism
The high up-regulation for ORF5508 (Ribulose-bisphosphate carboxylase, Rubisco), ORF4921 (Phosphoglycerate mutase, PGAM) and ORF4691 (Transketolase) was observed at 15°C. In contrast, ORF4691 (Transketolase), ORF3807 (Glyceraldehyde-3-phosphate dehydrogenase, GAPDH) and ORF2124 (Carbohydrate-selective porin) were the specific proteins up-regulated at 45°C. Transketolase is involved in both the reductive pentose phosphate cycle (RPPC) and the oxidative pentose phosphate cycle (OPPC). Ndong et al. [49] found that transketolase was expressed to a higher level when growing at 5°C compared to 20°C in winter rye. This is consistent with reports that growth and development of wheat, rye and Arabidopsis at low temperatures induce a general increase in the activities and levels of specific enzymes of RPPC and the sucrose biosynthesis pathway. Additionally, it has been proposed that the transketolase reaction may be considered near-limiting in the homeostatic regulation of carbon flux through the RPPC [49]. GAPDH is a key enzyme in the reductive pentose phosphate cycle. The cold adaptation of the lactic acid bacterium Lactococcus lactis results in disruption of sugar metabolism. In Lactobacillus rhamnosus HN001, GAPDH is not induced upon cold shock, but it is up-regulated when prestressed with heat (50°C) [50]. To date, few studies have examined the correlation of carbohydrate-selective porin at low temperatures.
(4): Translation, ribosomal structure and biogenesis
Under the low and high temperature cultivated conditions, there were 6 genes with up-regulated expression: ORF4248 (Cysteinyl-trna synthetase), ORF4125 (Tyrosyl-trna synthetase) and ORF1804 (Hypothetical protein) for the 15°C treatment, and ORF0073 (Ribosomal protein L6), ORF1074 (spot 433, Elongation factor TS) and ORF1074 (spot 514, Elongation factor TS) for the 45°C treatment. The above genes strongly expressed a series of proteins to alleviate the damage of high or low temperatures through participation in new peptide production, transportation, folding, assembly, positioning, refolding and degradation of denatured proteins [51].
(5): Coenzyme metabolism
At 15°C, the 5 differentially expressed proteins were involved in 4 up-regulated genes (ORF5578 (UBA/THIF-type NAD/FAD binding protein), ORF4133 (pyridoxal phosphate biosynthetic protein), ORF1829 (radical SAM domain-containing protein) and ORF4139 (tetrahydrofolatede hydrogenase). However, the 4 genes did not express at 45°C. Their preferential expression at low temperature prompted formation of some gene products, which were not expressed in the control, such as the DEAD box RNA helicase (crhR), fatty acid desaturation enzyme, RNA molecular chaperones, RNA binding protein, proline and NADH dehydrogenase subunit [45]. These substances can inhibit ice crystal formation, maintain osmotic balance, antioxidant activity and systematic stability of cell membranes, and thus they can enhance low temperature tolerance in ASP [52].
(6): Unassigned Functional genes
Additionally, we detected expression of 7 (15 °C treatment) and 8 genes (45°C treatment) with unassigned functions that were up-regulated at remarkably high levels (Table S3C). These genes with unknown functions may play an important role in resistance to high and low temperatures in ASP. Therefore, it is of great importance to predict the role of these unknown candidate genes induced as a temperature response. The expression of some genes with unknown functions was found to be appreciably down-regulated, such as ORF253, ORF4030 and ORF0361. Therefore, it is necessary to further study the expression regulation mechanisms of these genes at the transcriptional level under abnormal temperature conditions.
(7): The other bacterial genes
The 6 proteins not having high homology in ASP (YP_002248469, YP_002485086, ZP_04040440, YP_001999788, YP_912068, ZP_05256482) did have high homology with other prokaryotes, which belonged to Hermodesulfovibrio yellowstonii, Cyanothece sp. PCC 7425, Meiothermus ruber DSM 1279, Mycoplasma arthritidis, Chlorobium phaeobacteroides and Bacteroides, respectively. All of the above prokaryotes were associated with high temperature-tolerant bacteria, with the exception of Cyanothece sp. PCC 7425. These results suggest that the 6 proteins come from the target algal strains, not from contamination of other bacteria. In addition, gene “transfer or appropriate” phenomenon occurred in the 6 genes due to long-term environmental adaptation. Perhaps these genes play an important role in tolerating abnormal temperatures.
Some ASP genes, showing inconsistency between transcription and translation levels, were not directly associated with heat and cold tolerance, and perhaps their main roles are regulatory effects [53]. Regulation of gene expression can be controlled by structural gene activation, initiation of transcription, post-transcriptional processing and transport, mRNA degradation, translation and post-translational processing and protein degradation. The degradation of mRNA transcripts is a possible reason for the inconsistency between transcription and translation levels. Some inducible gene transcripts can be degraded immediately after translation and even in the course of translation [45]. The inconsistency between transcription and translation levels is influenced by many factors, and thus further investigation is required to elucidate its detailed mechanisms.
Obviously, the temperature-tolerant mechanism of ASP is not solely mediated through up- or down-regulation of one metabolite or one metabolic pathway, but through complex interactions from cross-regulation of many physiological, biochemical and metabolic pathways. In conclusion, we identified several interesting genes of ASP that responded to high or low temperature treatment by proteomic analysis. To elucidate the temperature-tolerant mechanisms in ASP, the data from this study will guide us to further investigate the expression regulation of these target genes.
Supporting Information
The differentially expressed protein profiles of 2-DE of ASP-YZ at different culture temperatures. Note: 1A- at 35°C; 1B- at 15°C; 1C- at 45°C.
(DOC)
The mass spectrum of identified protein spots.
(DOC)
The AGE profiles of total RNA in 15°C, 35°C and 45°C temperature treatments. Note: Lane 1, 15°C; Lane 2, control; Lane 3, 45°C treatment.
(DOC)
The primers used for amplification of target and 16S rRNA genes.
(DOC)
Annealing and extension temperatures for amplification of the target and 16S rRNA genes.
(DOC)
A The differentially expressed proteins between 15°C and 35°C treatments.
Note: (1) In “Fold change” column, “+”indicates the visible protein spots in the 15°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 15°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 35°C control group, and the negative fold indicates those with >3-fold down-regulation in the 15°C treatment group.
(3) The filled grayness indicates the other 6 genes have high homology with other microorganisms.
B The differentially expressed proteins between 45 °Cand 35 °C treatments.
Note: (1) In “Fold change” column, “+”indicates the visible protein spots in the 45°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 45°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 45°C treatment group, and the negative fold indicates those with >3-fold down-regulation in the 45°C treatment group.
C The differentially expressed proteins between 15°C and 45°C treatments.
Note: (1) In “Fold change” column, “+15” indicates the solely visible spots in the 15°C treatment gels, and “+45” indicates the solely visible protein spots in the 45°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 15°C treatment when compared to the 45°C treatment.
(DOC)
Funding Statement
This work was jointly funded by National Natural Science Foundation of China (31071115 and 31270548) and Key Project of Environmental Protection Department of Zhejiang Province, China (2012B014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pulz O, Gross W (2004) Valuable products from biotechnology of microalgae. Appl Microbiol Biotechnol 65: 635-648. doi: 10.1007/s00253-004-1647-x. PubMed: 15300417. [DOI] [PubMed] [Google Scholar]
- 2. Archana K, Anish Z, Urmila J, Pratiksha B (2008) Spirulina in health care management. Curr Pharm Biotechnol 9: 400-405. doi: 10.2174/138920108785915111. PubMed: 18855693. [DOI] [PubMed] [Google Scholar]
- 3. Hecker M , Schumann W, Völker U (1996) Heat-shock and general stress response in Bacillus subtilis . Mol Microbiol 19: 417-428. doi: 10.1046/j.1365-2958.1996.396932.x. PubMed: 8830234. [DOI] [PubMed] [Google Scholar]
- 4. Fang F, Barnum SR (2004) Expression of the heat shock gene hsp16.6 and promoter analysis in the cyanobacterium, Synechocystis sp. PCC 6803. Curr Microbiol 49: 192-198. PubMed: 15386103. [DOI] [PubMed] [Google Scholar]
- 5. Ehling-Schulz M, Schulz S, Wait R, Angelika G, Scherer S et al. (2002) The UV-B stimulon of the terrestrial cyanobacterium Nostoc commune comprises early shock proteins and late acclimation proteins. Mol Microbiol 46: 827-843. doi: 10.1046/j.1365-2958.2002.03209.x. PubMed: 12410839. [DOI] [PubMed] [Google Scholar]
- 6. Kojima K, Nakamoto H (2005) Post-transcriptional control of the cyanobacterial HspA heat-shock induction. Biochem Biophys Res Commun 331: 583-588. doi: 10.1016/j.bbrc.2005.04.009. PubMed: 15850800. [DOI] [PubMed] [Google Scholar]
- 7. Kurdrid P, Phuengcharoen P, Cheevadhanarak S, Tanticharoen M, Hongsthong1 A (2010) Identification of a heat shock-responsive cis-acting DNA sequence and its transcriptional regulator: Their roles in the expression of the Spirulina-desD gene in response to heat stress. J Biosci Bioeng 109: 205-210. doi: 10.1016/j.jbiosc.2009.09.002. PubMed: 20159564. [DOI] [PubMed] [Google Scholar]
- 8. Kojima K, Nakamoto H (2002) Specific binding of a protein to a novel DNA element in the cyanobacterial small heat-shock protein gene. Biochem Biophys Res Commun 297: 616-624. doi: 10.1016/S0006-291X(02)02256-8. PubMed: 12270139. [DOI] [PubMed] [Google Scholar]
- 9. Balogi Z, Cheregi O, Giese KC, Juhász K, Vierling E et al. (2008) A mutant small heat shock protein with increased thylakoid association provides an elevated resistance against UV-B damage in Synechocystis 6803. J Biol Chem 283: 22983-22991. doi: 10.1074/jbc.M710400200. PubMed: 18574246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kurdrid P, Senachak J, Sirijuntarut M, Yutthanasirikul R, Phuengcharoen P et al. (2011) Comparative analysis of the Spirulina platensis subcellular proteome in response to low- and high-temperature stresses: Uncovering cross-talk of signaling components. Proteome Sci 9: 39-55. doi: 10.1186/1477-5956-9-39. PubMed: 21756373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deshnium P, Paithoonrangsarid K, Suphatrakul A, Meesapyodsuk D, Tanticharoen M, et al. (2000) Temperature-independent and -dependent expression of desaturase genes in filamentous cyanobacterium Spirulina platensis strain C1 (Arthrospira sp. PCC 99438). FEMS Microbiol Lett 184: 207-213. [DOI] [PubMed] [Google Scholar]
- 12. Hongsthong A, Sirijuntarut M, Prommeenate P, Lertladaluck K, Porkaew K et al. (2008) Proteome analysis at the subcellular level of the cyanobacterium Spirulina platensis in response to low-temperature stress conditions. FEMS Microbiol Lett 288: 92-101. doi: 10.1111/j.1574-6968.2008.01330.x. PubMed: 18764876. [DOI] [PubMed] [Google Scholar]
- 13. Morsy FM, Nakajima M, Yoshida T, Fujiwara T, Sakamoto T et al. (2008) Subcellular localization of ferredoxin-NADP(+) oxidoreductase in phycobilisome retaining oxygenic photosynthetic organisms. Photosynth Res 95: 73-85. PubMed: 17828614. [DOI] [PubMed] [Google Scholar]
- 14. Li H, Yang S, Xie J, Feng J, Gong Y et al. (2007) The origin of the temperature-induced fluorescence fluctuation in Spirulina platensis: Temperature-sensitive mobility of PQ molecules. Photosynth Res 94: 59-65. doi: 10.1007/s11120-007-9214-9. PubMed: 17638113. [DOI] [PubMed] [Google Scholar]
- 15. Wu S, He L, Shen R, Zhang X, Wang Q (2011) Molecular cloning of maltooligosyltrehalose trehalohydrolase gene from Nostoc flagelliforme and trehalose-related response to stresses. J Microbiol Biotechnol 21: 830-837. doi: 10.4014/jmb.1101.10068. PubMed: 21876373. [DOI] [PubMed] [Google Scholar]
- 16. Richaond A, Boca R (1986) Handbook of microalgal mass culture. Microa Econ Potential: 199-244. [Google Scholar]
- 17. Giavalisco P, Nordhoff E, Lehrach H, Gobom J, Klose J (2003) Extraction of proteins from plant tissues for two-dimensional electrophoresis analysis. Electrophoresis 24: 207-216. doi: 10.1002/elps.200390016. PubMed: 12652593. [DOI] [PubMed] [Google Scholar]
- 18. Blum H, Beier H, Gross HJ (1987) Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8: 93-99. doi: 10.1002/elps.1150080203. [DOI] [Google Scholar]
- 19. Grove H, Faergestad EM, Hollung K, Martens H (2009) Improved dynamic range of protein quantification in silver-stained gels by modelling gel images over time. Electrophoresis 30: 1856-1862. doi: 10.1002/elps.200800568. PubMed: 19517441. [DOI] [PubMed] [Google Scholar]
- 20. Zhang YM, Zhao JM, Xiang Y, Bian XC, Zuo QM et al. (2011) Proteomics study of changes in soybean lines resistant and sensitive to Phytophthora sojae . Proteome Sci 9: 52-64. doi: 10.1186/1477-5956-9-52. PubMed: 21899734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang L, Yu ZF, Jiang L, Jiang J, Luo HB et al. (2011) Effect of post-harvest heat treatment on proteome change of peach fruit during ripening. J Proteomics 74: 1135-1145. doi: 10.1016/j.jprot.2011.04.012. PubMed: 21550427. [DOI] [PubMed] [Google Scholar]
- 22. Li W, Gao H, Yin C, Xu X (2012) Identification of a novel thylakoid protein gene involved in cold acclimation in cyanobacteria. Microbiology 158: 2440-2449. doi: 10.1099/mic.0.060038-0. PubMed: 22767544. [DOI] [PubMed] [Google Scholar]
- 23. Rhee JS, Ki JS, Kim BM, Hwang SJ, Choi IY et al. (2012) HspA and HtpG enhance thermotolerance in the cyanobacterium, Microcystis aeruginosa NIES-298. J Microbiol Biotechnol 22: 118-1125. doi: 10.4014/jmb.1108.08001. PubMed: 22297228. [DOI] [PubMed] [Google Scholar]
- 24. Barthel S, Rupprecht E, Schneider D (2011) Thermostability of two cyanobacterial GrpE thermosensors. Plant Cell Physiol 52: 1776-1785. doi: 10.1093/pcp/pcr116. PubMed: 21865302. [DOI] [PubMed] [Google Scholar]
- 25. Pinto F, Pacheco CC, Ferreira D, Moradas-Ferreira P, Tamagnini P (2012) Selection of suitable reference genes for RT-qPCR analyses in cyanobacteria. PLOS ONE, 7: e34983 PubMed: 22496882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karp NA, Lilley KS (2009) Investigating sample pooling strategies for DIGE experiments to address biological variability. Proteomics 9: 388-397. doi: 10.1002/pmic.200800485. PubMed: 19105178. [DOI] [PubMed] [Google Scholar]
- 27. Kurreck J (2009) RNA interference: From basic research to therapeutic applications. Angew Chem Int Ed Engl 48: 1378-1398. doi: 10.1002/anie.200802092. PubMed: 19153977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kainkaryam RM, Bruex A, Gilbert AC, Schiefelbein J, Woolf PJ (2010) pooIMC: smart pooling of mRNA samples in microarray experiments. BMC Bioinformatics 11: 299-310. doi: 10.1186/1471-2105-11-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Engel M, Behnke A, Bauerfeld S, Bauer C (2012) Sample pooling obscures diversity patterns in intertidal ciliate community composition and structure. FEMS Microbiol Ecol 79: 741-750. doi: 10.1111/j.1574-6941.2011.01255.x. PubMed: 22093023. [DOI] [PubMed] [Google Scholar]
- 30. Pronk TE, Ezendam J, Van Loveren H, Pennings JL (2011) Effects of pooling RNA from samples treated with different compounds for determining class specific biomarkers and processes in toxicogenomics. Toxicol in Vitro 25: 1841-1847. doi: 10.1016/j.tiv.2011.05.012. PubMed: 21620947. [DOI] [PubMed] [Google Scholar]
- 31. Mikulski A, Bernatowicz P, Grzesiuk M, Kloc M, Pijanowska J (2011) Differential levels of stress proteins (HSPs) in male and female Daphnia magna in response to thermal stress: A consequence of sex-related behavioral differences. J Chem Ecol 37: 670-676. doi: 10.1007/s10886-011-9969-5. PubMed: 21614533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bradbury LM, Shumskaya M, Tzfadia O, Wu SB, Kennelly EJ et al. (2012) Lycopene cyclase paralog CruP protects against reactive oxygen species in oxygenic photosynthetic organisms. Proc Natl Acad Sci U S A 109: 1888-1897. doi: 10.1073/pnas.1206002109. PubMed: 22706644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zeng X, Wen P, Chen G (2012) Stress response of Synechocystis sll0862 mutant to heat shock and oxidative stress. J Microbiol 52: 594-601 (in Chinese). [PubMed] [Google Scholar]
- 34. Sheng J, Kim HW, Badalamenti JP, Zhou C, Sridharakrishnan S et al. (2011) Effects of temperature shifts on growth rate and lipid characteristics of Synechocystis sp. PCC6803 in a bench-top photobioreactor. Bioresour Technol 102: 11218-11225. doi: 10.1016/j.biortech.2011.09.083. PubMed: 22001056. [DOI] [PubMed] [Google Scholar]
- 35. Amiri RM, Yur'eva NO, Shimshilashvili KR, Goldenkova-Pavlova IV, Pchelkin VP et al. (2010) Expression of acyllipid Delta12-desaturase gene in prokaryotic and eukaryotic cells and its effect on cold stress tolerance of potato. J Integr Plant Biol 52: 289-297. doi: 10.1111/j.1744-7909.2010.00890.x. PubMed: 20377689. [DOI] [PubMed] [Google Scholar]
- 36. Apiradee H, Matura S, Rayakorn Y (2009) Subcellular proteomic characterization of the high-temperature stress response of the cyanobacterium Spirulina platensis . Proteome Sci 7: 35-36. doi: 10.1186/1477-5956-7-35. PubMed: 19737410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Apiradee H, Matura S, Rayakorn Y, Jittisak S, Pavinee K et al. (2000) Involvement of DnaK3, one of the three DnaK proteins of cyanobacterium Synechococcus sp. PCC in translational process on the surface of the thylakoid membrane. Biosci Biotechnol, Biochem 70: 1592-1598. [DOI] [PubMed] [Google Scholar]
- 38. Samuel P, Vadhanal AKP, Kamatchi R, Antony A, Meenakshisundaram S (2013) Effect of molecular chaperones on the expression of Candida antarctica lipase B in Pichia pastoris . Microbiol Res 168: 615-620. doi: 10.1016/j.micres.2013.06.007. PubMed: 23871144. [DOI] [PubMed] [Google Scholar]
- 39. Giró M, Ceccoli RD, Poli HO, Carrillo N, Lodeyro FA (2011) An in vivo system involving co-expression of cyanobacterial flavodoxin and ferredoxin–NADP+ reductase confers increased tolerance to oxidative stress in plants. FEBS Open Biol 1: 7-13. doi: 10.1016/j.fob.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dikanov SA, Samoilova RI, Kappl R, Crofts AR, Hüttermann J (2009) The reduced [2Fe-2S] clusters in adrenodoxin and Arthrospira platensis ferredoxin share spin density with protein nitrogens, probed using 2DE. Phys Chem 11: 6807-6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. MacColl R (1998) Progress toward complete structural and functional analysis via molecular genetics. J Struct Biol 124: 311-334. doi: 10.1006/jsbi.1998.4062. PubMed: 10049814. [DOI] [PubMed] [Google Scholar]
- 42. Mohanty N, Murthy SDS, Mohanty P (1987) Reversal of heat induced alterations in photochemical activities in wheat primary leaves. Photosynth Res 14: 259-267. doi: 10.1007/BF00032709. [DOI] [PubMed] [Google Scholar]
- 43. Gao X, Wei TD, Zhang N, Xie BB, Su HN et al. (2012) Molecular insights into the terminal energy acceptor in cyanobacterial phycobilisome. Mol Microbiol 85: 907-915. doi: 10.1111/j.1365-2958.2012.08152.x. PubMed: 22758351. [DOI] [PubMed] [Google Scholar]
- 44. Kirilovsky D, Kerfeld CA (2013) The orange carotenoid protein: A blue-green light photoactive protein. Photochem Photobiol Sci 12: 1135-1143. doi: 10.1039/c3pp25406b. PubMed: 23396391. [DOI] [PubMed] [Google Scholar]
- 45. Wada H, Hirasawa R, Omata T, Murata N (1984) The lipid phase thylakoid and cytoplasmic membranes from the blue green algae (Cyanobacteria), Anacystis nidulans and Anabaena variabilis . Plant Cell Physiol 25: 907-911. [Google Scholar]
- 46. Jonas J (1990) Cold denaturation of proteins. CRC Crit Rev Biochem 25: 281-305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 47. Akimoto S, Yokono M, Aikawa S, Kondo A (2013) Modification of energy-transfer processes in the cyanobacterium, Arthrospira platensis, to adapt to light conditions, probed by time- resolved fluorescence spectroscopy. Photosynth Res [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 48. Schluchter WM, Shen G, Alvey RM, Biswas A, Saunée NA et al. (2010) Phycobiliprotein biosynthesis in cyanobacteria: Structure and function of enzymes involved in post-translational modification. Adv Exp Med Biol 675: 211-228. doi: 10.1007/978-1-4419-1528-3_12. PubMed: 20532743. [DOI] [PubMed] [Google Scholar]
- 49. Ndong C, Danyluk J, Huner NP, Sarhan F (2001) Survey of gene expression in winter rye during changes in growth temperature, irradiance or excitation pressure. Plant Mol Biology 45: 691-703. doi: 10.1023/A:1010684719225. [DOI] [PubMed] [Google Scholar]
- 50. Prasad J, McJarrow P, Gopal P (2003) Heat and osmotic stress responses of probiotic Lactobacillus rhamnosus HN001 (DR20) in relation to viability after drying. Appl Environ Microbiol 69: 917-925. doi: 10.1128/AEM.69.2.917-925.2003. PubMed: 12571012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Szabados L, Kovcs H, Zilberstein A (2011) Plants in extreme environments: Importance of protective compounds in stress tolerance. Adv Bot Res 57: 105-150. doi: 10.1016/B978-0-12-387692-8.00004-7. [DOI] [Google Scholar]
- 52. Rosana AR, Ventakesh M, Chamot D, Patterson-Fortin LM, Tarassova O et al. (2012) Inactivation of a low temperature-induced RNA helicase in Synechocystis sp. PCC 6803: Physiological and morphological consequences. Plant Cell Physiol 53: 646-658. doi: 10.1093/pcp/pcs020. PubMed: 22368073. [DOI] [PubMed] [Google Scholar]
- 53. Matsuda N, Uozumi N (2006) Ktr-mediated potassium transport, a major pathway for potassium uptake, is coupled to a proton gradient across the membrane in Synechocystis sp. PCC 6803. Biosci Biotechnol, Biochem 70: 273-275. doi: 10.1271/bbb.70.273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The differentially expressed protein profiles of 2-DE of ASP-YZ at different culture temperatures. Note: 1A- at 35°C; 1B- at 15°C; 1C- at 45°C.
(DOC)
The mass spectrum of identified protein spots.
(DOC)
The AGE profiles of total RNA in 15°C, 35°C and 45°C temperature treatments. Note: Lane 1, 15°C; Lane 2, control; Lane 3, 45°C treatment.
(DOC)
The primers used for amplification of target and 16S rRNA genes.
(DOC)
Annealing and extension temperatures for amplification of the target and 16S rRNA genes.
(DOC)
A The differentially expressed proteins between 15°C and 35°C treatments.
Note: (1) In “Fold change” column, “+”indicates the visible protein spots in the 15°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 15°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 35°C control group, and the negative fold indicates those with >3-fold down-regulation in the 15°C treatment group.
(3) The filled grayness indicates the other 6 genes have high homology with other microorganisms.
B The differentially expressed proteins between 45 °Cand 35 °C treatments.
Note: (1) In “Fold change” column, “+”indicates the visible protein spots in the 45°C treatment gels, while not visible in the 35°C control gels. “-” indicates the visible protein spots in the 35°C control gels, while not visible in the 45°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 45°C treatment group, and the negative fold indicates those with >3-fold down-regulation in the 45°C treatment group.
C The differentially expressed proteins between 15°C and 45°C treatments.
Note: (1) In “Fold change” column, “+15” indicates the solely visible spots in the 15°C treatment gels, and “+45” indicates the solely visible protein spots in the 45°C treatment gels.
(2) The positive fold indicates the protein spots with >3-fold up-regulation in the 15°C treatment when compared to the 45°C treatment.
(DOC)