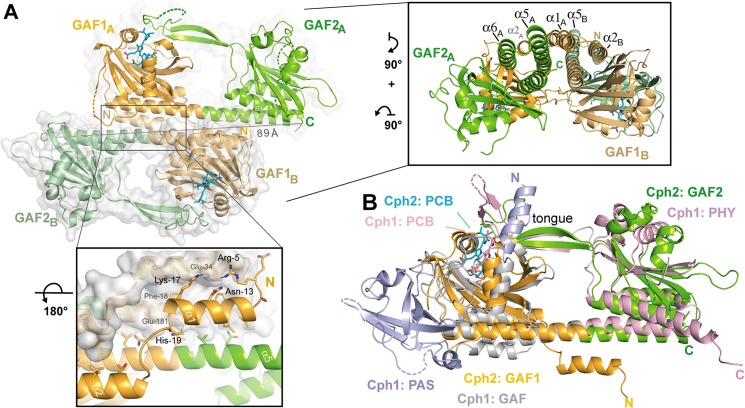
FIGURE 3.
Quaternary structure of the knotless SynCph2(1–2) photosensor and comparison to SynCph1. A, the antiparallel dimer of SynCph2(1–2). The distance between C-terminal residues His421 (molecule A) and Ile418 (molecule B) is 89 Å; lower inlet, the N-terminal helix forms part of the dimer interface; inlet on the right side, perpendicular view on the SynCph2(1–2) dimer. The interface between the monomers is built of a helix bundle composed of the linker α-helix and shorter helices, especially the N-terminal helix. B, structural superposition of SynCph2 and SynCph1 (r.m.s. deviation 2.64 Å for 249 Cα atoms); the PAS, GAF, and PHY domains of SynCph1 are depicted in blue, gray, and pale red, respectively.