FIGURE 3.
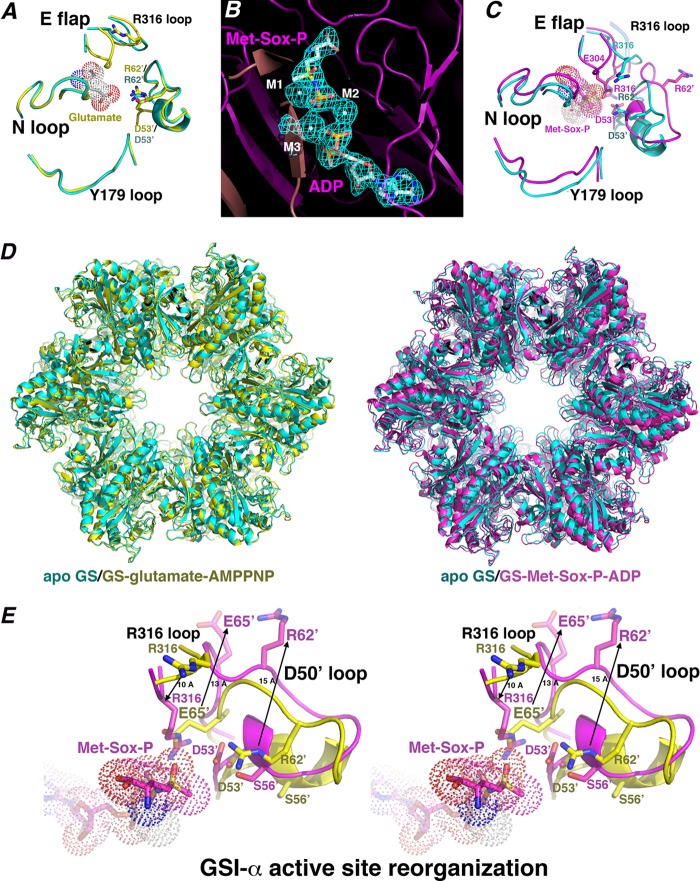
B. subtilis GS active site architecture, substrate binding, and transition state conformational changes. A, close-up of key active site loop regions in the apo-GS (cyan) and glutamate-bound GS structures (yellow). The apo-Glu flap is mostly disordered, whereas in the glutamate-bound structure, the Glu flap backbone atoms are resolved. No large structural changes are noted between key loops. B, initial Fo − Fc map of the GS·transition state complex contoured at 3.5 σ calculated before the addition of Met-Sox-P, ADP, and metal ions (to 2.58 Å). C, close-up of the active site regions around key loops (as in A) after superimposition of the apo-state (cyan) and transition state (magenta) complexes. Large structural changes in the loops occur upon transition state formation, especially in the Asp50′ loop. D, superimposition of the apo-GS dodecamer (cyan) onto the glutamate·AMPPCP-bound (yellow) and Met-Sox-P·ADP-bound (magenta) forms. E, stereo view showing superimposition of the substrate-bound form of GS (yellow) and the transition state complex (magenta), highlighting critical active site loop rearrangements that provide proper active site architecture for catalysis during formation of the transition state. Most notable are the complete ejection of Arg62′ and Glu65′, which permit adjustment of Asp53′ and Arg316 into the pocket for catalysis. The extent of the structural changes is indicated by arrows and distances in Å.