FIGURE 4.
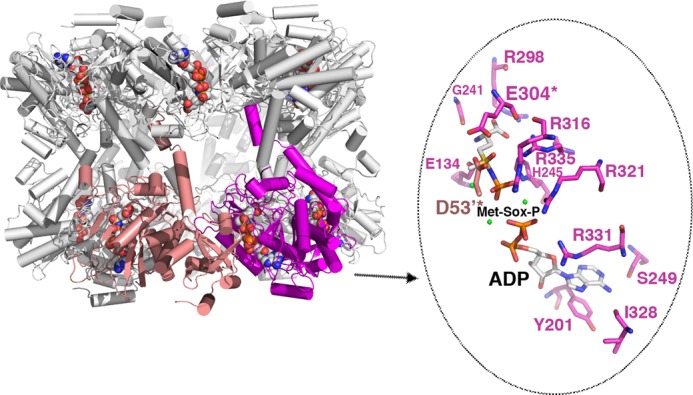
Structure of the GSI-α Met-Sox-P·ADP complex and close-up of contacts to the transition state. The structure of the dodecamer is displayed as ribbons with one subunit colored magenta and the neighboring subunit colored salmon. The Met-Sox-P and ADP molecules in each active site are shown as CPK. Note that there is a dodecamer in the crystallographic asymmetric unit, and each active site (formed between subunits) has generated the identical transition state with the same contacts. One active site (between the magenta and salmon subunits) is highlighted, and key residues that contact Met-Sox-P and ADP are shown as sticks (enlarged view to the right). The key catalytic residues Asp53 and Glu304 are labeled and highlighted with asterisks. Magnesium ions are colored green.
