FIGURE 6.
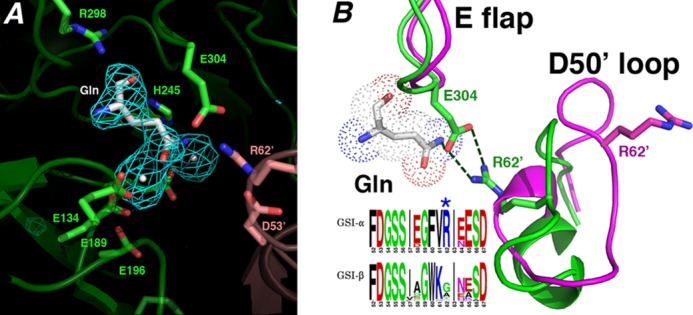
Structural basis for GSI-α-specific Gln feedback inhibition. A, initial Fo − Fc map calculated before the addition of glutamine and metal ions and contoured at 3.2 σ (to 2.95 Å). B, superimposition of active site loops of the glutamine-bound state (green) and the transition state complex (magenta). The Gln-bound active site loops adopt structures similar to apo- and substrate-bound except that the Glu flap (E flap) is well ordered, and the Glu flap residue Glu304 hydrogen-bonds to the Gln as well as the GSI-α-specific residue from the Asp50′ loop (D50′ loop), Arg62′. Notably, GSI-β enzymes are not feedback-inhibited by Gln. As shown by the logo, GSI-β enzymes all lack the key Arg62′ residue, explaining why they are not subject to Gln feedback inhibition (in the logo, residues are colored according to type, basic in blue, acidic in red, hydrophobic in black, and glycine/serine in green).
