FIGURE 7.
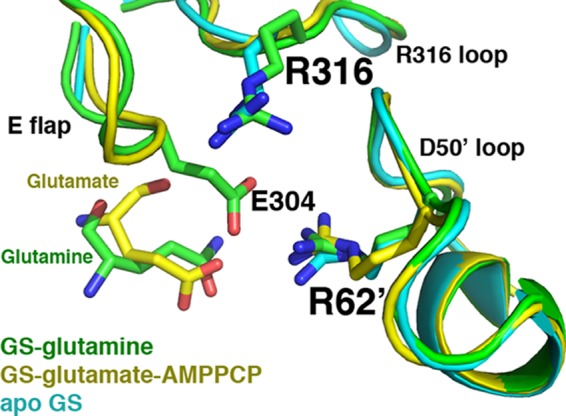
The GS·glutamine structure active site is similar to that of the apo- and substrate-bound forms. Superimposition of the apo-, glutamate-AMPPCP, and glutamine-bound GSI-α structures, focusing on key active site loops. The apo-structure is colored cyan, the glutamate-AMPPCP-bound structure is yellow, and the glutamine-bound structure is green. The active site loops adopt essentially the same conformation. The Glu flap in the apo-state is mostly disordered, whereas backbone atoms of the Glu flap in the substrate-bound state are visible (but not side chains), and in the glutamine-bound form, the Glu flap is as well ordered as in the transition state.
