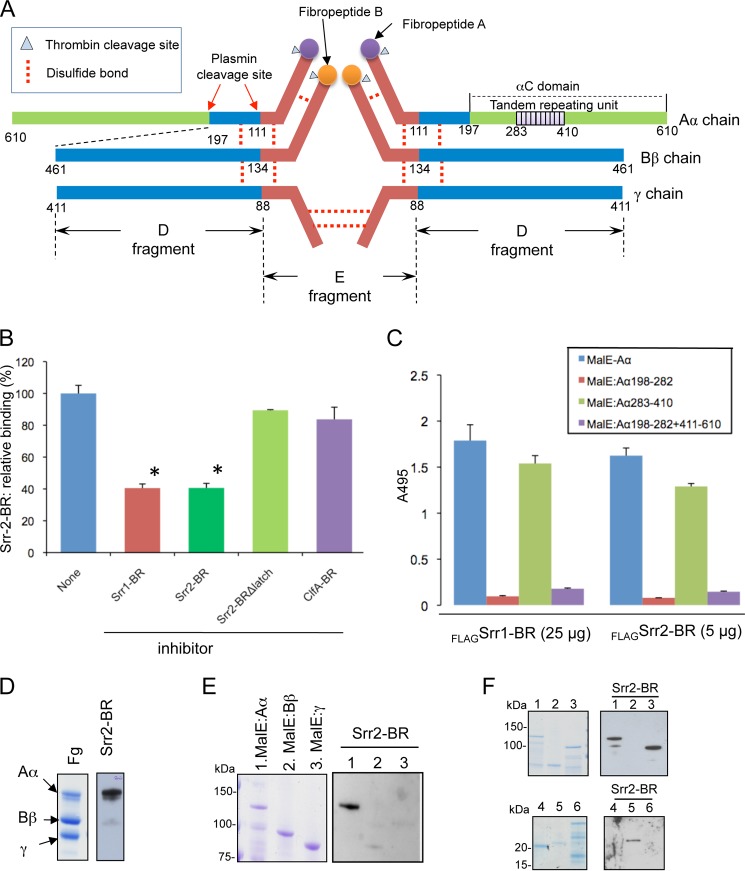
FIGURE 4.
Identification of the Srr2 binding domain on fibrinogen. A, schematic drawing of human fibrinogen. The 10 tandem repeating units of the Aα chain are shown in purple. B, inhibition of FLAG-Srr2-BR (0.05 μm) binding to immobilized fibrinogen by purified untagged proteins (10 μm). C, binding of FLAG-Srr1-BR (FLAGSrr1-BR) (25 μg/ml) or FLAG-Srr2-BR (FLAGSrr1-BR (5 μg/ml) to MBP fused with full-length recombinant Aα (MBP-Aα) or subdomains of the Aα chain. Subscripts indicate amino acids contained within each fragment. Bars represent the mean binding levels (± S.D.). D, Srr2-BR binding to the fibrinogen (Fg) Aα chain. Purified human fibrinogen was separated by SDS-PAGE and stained with Coomassie Blue (left panel). Far Western blotting of fibrinogen with Srr2-BR is shown in the right panel. E, recombinant MBP-Aα, Bβ, and γ chains probed with FLAG-Srr2-BR (5 μg/ml). F, recombinant MBP-Aα and its truncated variants probed with FLAG-Srr2-BR (5 μg/ml; right). Lane 1, MBP:Aα(1–610); lane 2, MBP:Aα(1–197); lane 3, MBP:Aα(198–610); lane 4, MBP:Aα(198–282); lane 5, MBP:Aα(283–410); and lane 6, MBP:Aα(198–282 + 411–610).