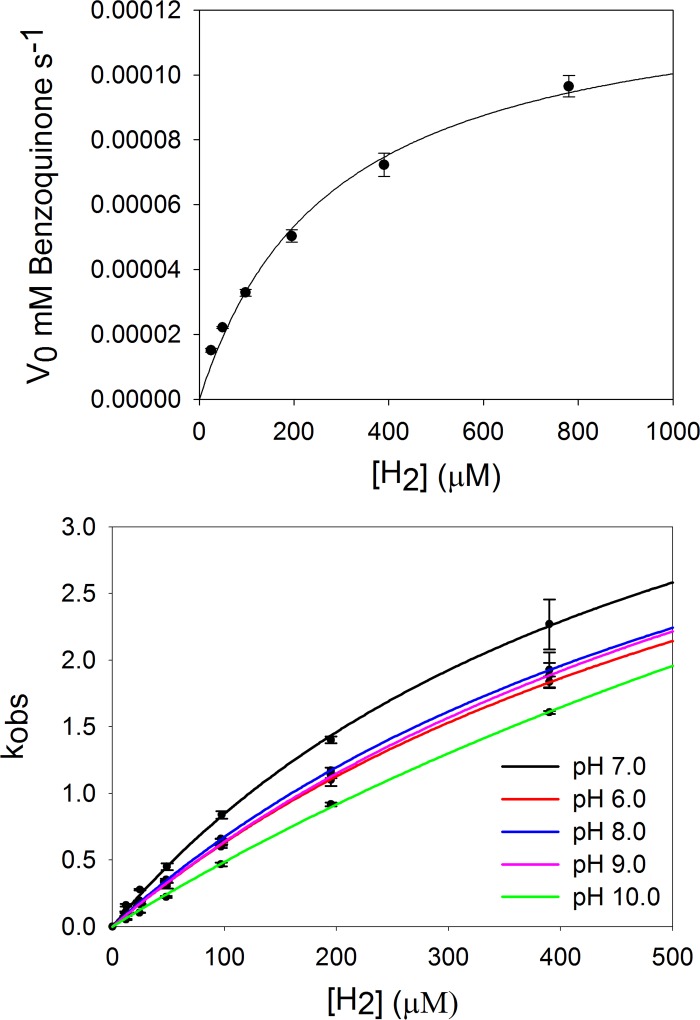
FIGURE 2.
Steady-state and rapid reaction kinetics of CO dehydrogenase with H2 as substrate. Top, steady-state H2 concentration dependence of CO dehydrogenase using 50 μm 1,4-benzoquinone as the electron acceptor in 50 mm HEPES, pH 7.2, at 25 °C. Plots of the initial rates, following 1,4-benzoquinone reduction at 247 nm after mixing a final concentration of 25 nm CO dehydrogenase with H2 dissolved in 1,4-benzoquinone, versus [H2] were fit with the Michaelis-Menten equation using Sigma Plot (R2 = 0.995) to give a kcat of 5.1 s−1 and Km of 283 μm. Bottom, rapid reaction kinetic plots of the rate kobs following the reduction of 5 μm CO dehydrogenase at 450 nm versus [H2], from 12 μm to 390 μm, at (50 mm phosphate, pH 6.0, 50 mm HEPES, pH 7.2, 50 mm Tris-HCl, pH 8.0 and pH 9.0, and 50 mm CAPS, pH 10.0), 25 °C. Plots were fit using Equation 1 to yield a rate of reduction and substrate dissociation constant, kred and Kd. The optimum pH, 7.2, gave kred of 5.3 s−1 and Kd of 525 μm (R2 = 0.999).