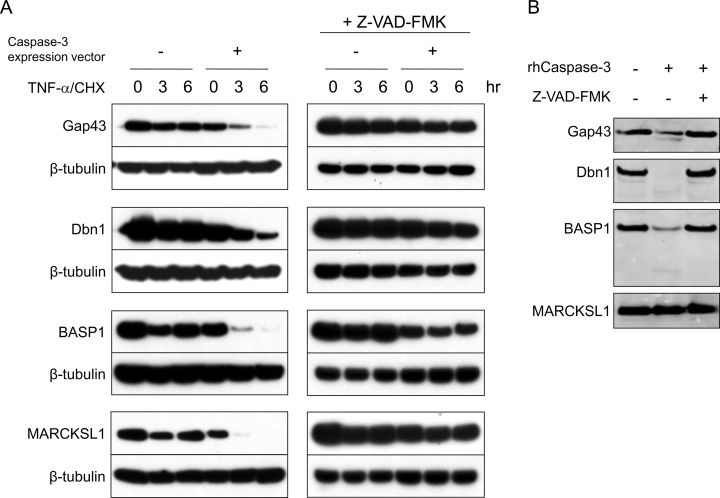
Fig. 3.
Caspase-3 cleavage assay for Gap43, Dbn1, BASP1, and MARCKSL1. A, In vivo caspase-3 cleavage assay. Hela cells were transfected for 4–5 h with constructs expressing V5-tagged candidate caspase-3 substrates alone (Gap43, Dbn1, BASP1, or MARCKSL1) or cotransfected with a construct expressing caspase-3. To confirm the reduction of the tested protein is because of caspase activation, the caspase inhibitor Z-VAD-FMK (200 μm) was applied to the medium throughout the same test right after transfection. About 16 h after transfection, the cells were treated with TNF-α and cycloheximide (CHX) to induce apoptosis. Cell lysates were collected at 0, 3, and 6 h after apoptosis induction for immunoblotting against V5. B, In vitro caspase-3 cleavage assay. The V5 tagged candidate genes were expressed in vitro. Synthesized proteins were incubated with recombinant active caspase-3 (50 ng) alone or along with the caspase inhibitor Z-VAD-FMK (200 μm) for 16 h at 37 °C, then analyzed by immunoblotting against V5.