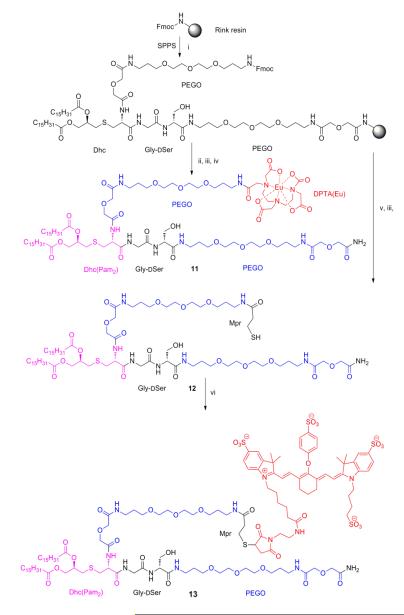
Scheme 1. Synthetic Route for Eu-DTPA Compound 11, Mpr Compound 12 and IRDye800CW Compound 13.
iFmoc/tBu synthesis continued as follows: a) Piperidine/DMF (1:4) for Fmoc deprotection; b) Fmoc-aa-OH (3eq), HOBt (3eq), DIEA (6eq), and HBTU (3eq) in DMF for amino acid couplings; ii DTPA was attached after Fmoc deprotection as follows: a) DTPA anhydride (3 eq) and HOBt (3 eq) were dissolved in dry DMSO (0.5M), heated to 60°C for 3 min then stirred at room temperature for 30 min, b) preformed DTPA-OBt diester mixture reacts with the resin overnight; iii TFA-scavenger cocktail (90% trifluoroacetic acid, 5% water, 5% triisopropylsilane) for 2 h; iv Eu(III)Cl3 (3.0 eq.) in 0.1M ammonium acetate buffer pH 8.0 overnight; v a) Piperidine/DMF (1:4) for Fmoc deprotection; b) Trt-3-mercaptopropionic acid (3eq), DIEA (6eq), and HBTU (3eq) in DMF; vi IRDye800CW maleimide (1 eq) in DMF.