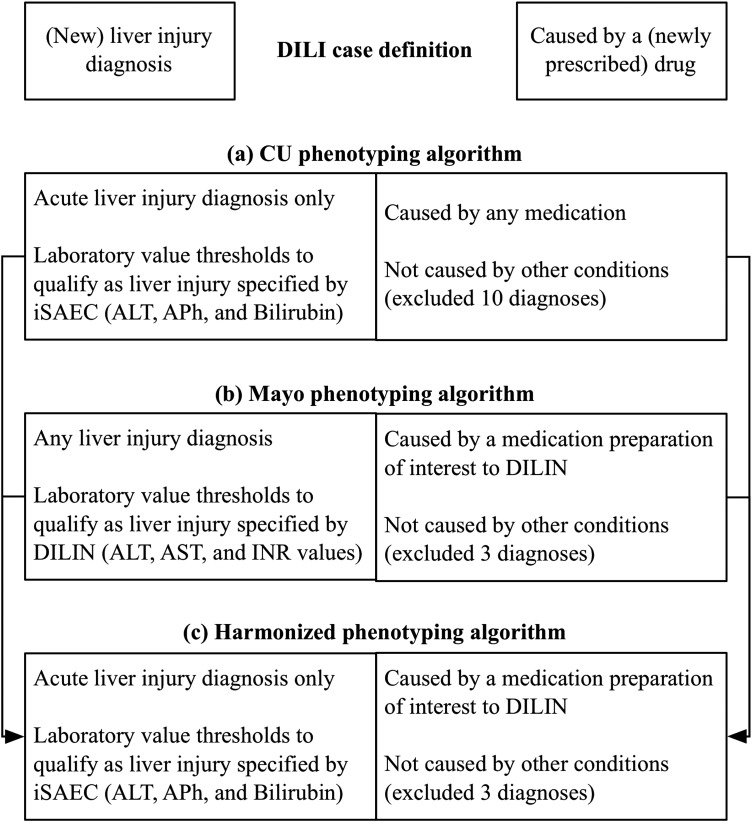
Figure 3.
Drug-induced liver injury (DILI) case definition and phenotyping algorithm harmonization. (A) Columbia University (CU) phenotyping algorithm: CU's International Serious Events Consortium (iSAEC)-informed algorithm makes a distinction between acute liver injury and chronic liver injury. CU chose to focus on acute liver injury. With respect to drug exposure, CU considered patients with any drug prescribed within 90 days of an acute liver injury diagnosis. Given iSAEC protocol specifications and access to structured data, CU considered iSAEC-specified threshold values for alanine aminotransferase (ALT), intestinal alkaline phosphatase (APh) and intervascular bilirubin. CU excluded 10 diagnoses initially. (B) Mayo Clinic (Mayo) phenotyping algorithm: Mayo's Drug Induced Liver Injury Network (DILIN)-informed algorithm considered any liver injury-related diagnoses. Mayo also considered a subset of drugs of interest to DILIN; and specified the temporal relationship between drug administration, DILI diagnosis and laboratory measures. Given DILIN protocol specifications and access to clinical notes of recruited patients, Mayo used DILIN-specified thresholds and text terms for ALT, aspartate aminotransferase (AST), and international normalized ratio (INR) for use by the cTAKES natural language processing engine. Specific emphasis was laid on investigating the DILI-related complications and medications in various sections of the clinical notes (including chief complaints, impression report plans). Mayo excluded three diagnoses initially. Given Mayo's focus on a smaller number of medications, exclusion criteria were less stringent than CU in order to optimize recall. (C) Harmonized phenotyping algorithm. See supplementary file 1 (available online only) for more detail.